Problems Set (Meeting 9)
General Announcements
1. Quiz 4
Quiz 4 will be administered on Tuesday, February 2, 2016 at 9
am
covering the gas chromatography and the spectroscopy of epoxides (MS,
NMR, etc.). Otherwise, you will not be allowed to take the quiz. Make
sure to bring a ruler and a non-graphing calculator with you.
2. Lidocaine Project
a. The postlab report for the lidocaine project has to be written in the lab notebook and submitted to the teaching assistant. The due dates are
February 11, 2016 or February 12, 2016 in your regular lab section. The report has to be written in the lab notebook and is worth 15 points.
3. Research project (Week 8/9)
a. Please look start looking for a partner for the Ferrocene project. Your partner should be in a different time slot than yourself i.e., if you are in the T/R afternoon section, try to find somebody in the W/F afternoon section. This way nobody has to put in additional time outside their regular schedule, which means that each group has four lab meetings to complete the project. We have to avoid as much as possible to have the entire class running around in the lab (Safety!).
Questions (due on
2/2/2016 or 2/3/2016)
1. Referring to the conversion of a-chloro-2,6-dimethylacetanilide to lidocaine (step 3), answer the following questions. Show pertinent balanced chemical equations.
a. Why is it important that the anilide is properly dried? How is this accomplished?
b. Which solvent is used in the reaction? Rationalize the choice.
c. Assuming that the student started with 1.00 g of the anilide, how much diethylamine is going to be used (in mL)?
d. The reaction mixture is refluxed. Which observations should the student make?
e. After the extraction with water, the organic layer is extracted several times with 3 M hydrochloric acid. In which layer is the product afterwards? In which form?
f. How is the lidocaine isolated from the solution obtained in e.? Provide a balanced equation for this reaction.
g. The student has to submit a sample for GC/MS analysis. Which solvent is used here? What is the desirable concentration?
2. Spartan assignment (Part 4 of the Project, can be done ooutside the lab or during meeting 10)
The goal of this exercise is to determine the barrier of rotation of an amide bond. To this end, the energy of the N,N-dimethylbenzamide (PhCON(CH3)2) is determined for dihedral angles from 0 to 360 degrees.
Instructions:
1. After drawing the molecule in Spartan 2014, click on Geometry-Constrain Dihedral.
2. Select the atoms shown below in the specific sequence C..N..C..O. A pink line will appear between N and C2. This will define two planes O1C3N1 and C3N1C5, which are going to be rotated towards each other in this part.

3. Click on the lock tool,
 , in the bottom right corner so that it looks like this,
, in the bottom right corner so that it looks like this,

4. Set the dihedral angle to 10 and hit the ENTER key.
5. Click on the minimize tool,
 , and wait for the operation to complete
, and wait for the operation to complete
6. Click on the minimize tool,
 , a second time and wait for the operation to complete.
, a second time and wait for the operation to complete.
7. Select Calculations from the Setup menu. The following window should appear. Select the options shown.
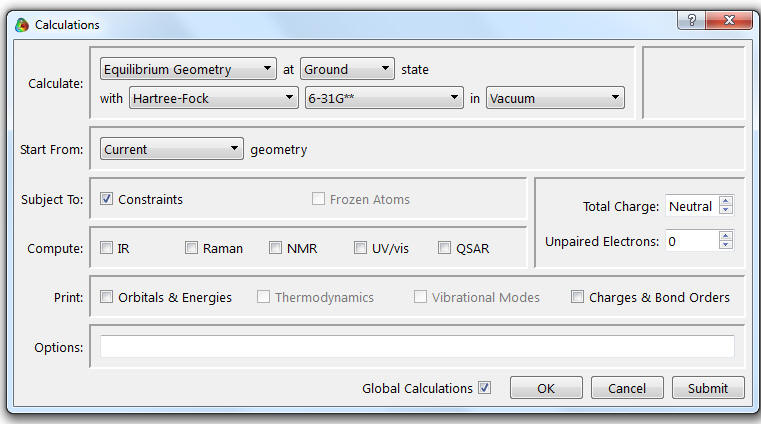
Pick Hartree-Fock (3-21) in vacuum.
8. Make sure the ![]() are checked. The Total Charge is Neutral and the Multiplicity is Singlet. All other boxes should be unchecked.
are checked. The Total Charge is Neutral and the Multiplicity is Singlet. All other boxes should be unchecked.
9. Click OK to close the window. Save the file with a unique name (i.e., DBA_0 degrees)
10. Select Submit from Setup menu. When the calculation is completed you will be notified.
11. Under the Display menu, select Properties.
12. A window should appear (the values in this example should differ from yours). If there are no values in the window then you need to double click on the molecule.
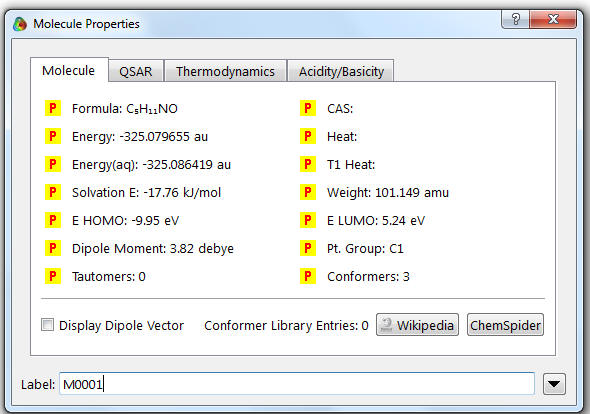
The energy of the molecule is recorded (here: -325.0079655 au).
13. Repeat this for all angles between 0 and 360 degree in 10 degree increments. (Hint: Just click on the pink bond and change the value in the box. Do not define a new constraint at this point because this will confuse the program completely.)
14. Using EXCEL, plot the energy of N,N-dimethylbenzamide vs. the dihedral angle (use MS Excel, XY Scatter plot). Rationalize the observed trends. Determine the barrier of rotation from the graph (1 au = 2625.5 kJ). How well does the calculated value match the data provided in the course reader?
15. Include the graph and the discussion of the data into the postlab report for the lidocaine project.