last updated
ATTN: Answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. Referring to the synthesis and characterization of the Jacobsen catalyst, answer the following questions.
a. Why is a slow air stream passed through the reaction mixture?
b. Why is heptane used duringt the work-up of the catalyst?
c. Which changes do you expect to observe in the IR spectrum of the Mn-complex (in comparison with the ligand)?
d. Why is the catalyst dark brown while the ligand is yellow?
e. What would be a good concentration to acquire the UV-Vis spectrum for teh catalyst? Which range should be measured?
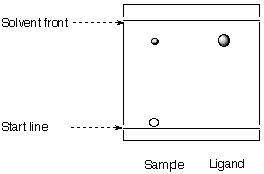
2. A student obtains the following TLC spectrum during the synthesis of the Jacobsen ligand.

a. Calculate the Rf-values.
b. Identify the individual spots. What can be said about the polarity of the ligand and the catalyst?
c. Which conclusion can the student draw about the progress of his reaction?
d. Why is a solvent mixture used for the TLC?
4.