Transition metals
Transition metals usually possess partially filled d- or f-shells. All of them are metals and they are often times paramagnetic. Most of their compounds are colored. Since they exhibit a broad variety of oxidation states e.g. manganese (- 3) to (+7), they have also become very important tools in various areas. They can be more readily oxidized and/or reduced than main group elements, which makes it possible to fine-tune a broad variety of properties by using different ligands.
| Compound | Color | Oxidation state |
| Mn2(CO)10 | yellow | 0 |
| Mn(CO)5Br | yellow orange | +1 |
| MnSO4 | pale pink | +2 |
| Mn(OAc)3 | brown | +3 |
| MnO2 | dark brown | +4 |
| Na3MnO4 | blue | +5 |
| K2MnO4 | green | +6 |
| KMnO4 | purple | +7 |
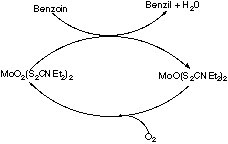
Many transition metal compounds ae used in organic chemistry as oxidants e.g. chromate, permanganate, OsO4, etc. In some cases, the oxidation can be performed catalytically using a transition metal complex and air as shown below.

Many TM halides are Lewis acids, which makes them useful tools in catalysis e.g. polymerization of alkenes (e.g. TiCl4), Friedel-Crafts-acylation (e.g. FeCl3) or bromination of aromatic rings (e.g. FeBr3).
Crystal Field Theory
If there are no ligands present, all d-orbitals are degenerated (possess the same energy). Once ligands are introduced to the system (by definition approaching along the x-, y- or z-axis), the orbitals split up depending on the degree of interaction of the electrons in the d-orbitals and the electrons of the ligand. Generally, repulsion leads to an increase in orbital energy.
In an octahedral geometry, the energies of the orbitals directly located on the axis (dx2-y2 and dz2) are raised while the orbitals in between the axis (dxy, dxz, dyz) are lowered. Overall, the system has to be balanced in a way that no energy is gained or lost if all orbitals are filled. The degree of splitting (Dq parameter) depends on the metal and the ligand surrounding it and is summarized in tables (nephelauxetic series). This data is usually obtained from the analysis of UV-Vis spectra. Generally, the allowed electronic transitions require energies that correspond to light in the visible range. Hence, most transition metal compounds are colored.
However, the situation becomes a little bit more complicated of the coordination around the metal is less symmetric e.g. trigonal byramidal or tetragonal pyramidal. The five d-orbitals split up in three (or even more) different energy levels
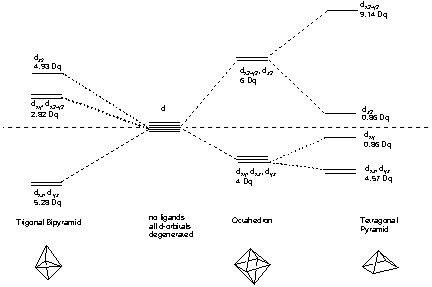
For open d-shells, there is often the chance of high-spin or low spin complexes. In addition, a distortion of the polyhedron can also lead to gain of stability (Jahn-Teller-Effect). This effect is often observed in d8-systems e.g. Pd2+ that often exert square planar geometry.
Cytochromes
Heme proteins often act a electron carrier to facilitate the oxidation of various substrates using molecular oxygen. This is possible because iron can assume a wide range of oxidation states (FeII-FeV) in those reactions. One of the most investigated systems is the monooxygenase cyctochrome P450. Iron exhibits a coordination number of six in a slightly distorted octahedral geometry (four nitrogen atoms from a porphyrin, one sulfur atom from the cysteine and one from the oxygen of water)
The catalytic cycle demonstrates the conversion of a C-H group to a C-OH group.
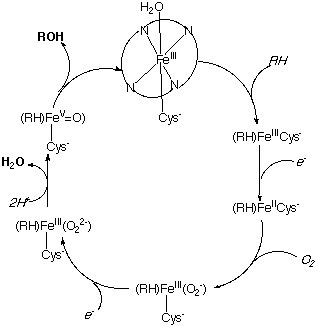
The overall reaction can be written as
![]()
Cobalamin
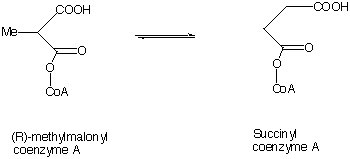
Vitamin B12 is required for several transformations such as the conversion of (R )-methylmalonyl co-enzyme A into succinyl co-enzyme A. One the important subunits in this vitamin is cobalamin. The human body contains ~5 mg.
Four nitrogen atoms of a macrocycle, one nitrogen atom from a side arm of the ligand and one carbon atom from the R-group result in an octahedral geometry aaround the cobalt atom.
The redox chemistry of cobalamin can be described by the following intermediates:
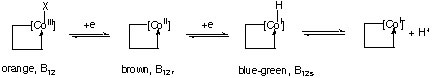
The reduced form is a very powerful reducing reagent that is capable to reduce nitrate to ammonium and chlorate to chloride.
Nitrogenases
Ammonia is currently produced using the Haber-Bosch process. However, this process requires very pure staring materials (hydrogen, nitrogen), high pressures (~270 atm), high temperature (450 C) and a iron catalyst and affords relative poor yields. Ammonium salts are used as fertilizers in big quantities. Ammonium nitrate (NH4NO3) can decompose rapidly to form N2O and H2O. It is therefore used as explosive.
In nature, nitrogenases from bacteria and blue-green algae perform this conversion in relative efficient ways. Over the past 40 years, chemists have tried to develop alternatives for these nitrogen-fixing systems. They synthesized nitrido and imido complexes of molybdenum, tungsten, rhenium and osmium as models for the conversion of molecular nitrogen to ammonia.
![]()
Even though progress has been made, as of today there is no commercially attractive alternative.