1. a. The ligand provides the asymmetric induction into the epoxidation reaction. The cyclohexane part in the catalyst introduces the asymmetric induction into the system because the bridge is asymmetric (C2-asymmetry) when the (R, R) or the (S, S)-enantiomer of the diamine is used. The meso form of 1,2-diaminocyclohexane is achiral would not lead to the formation of a racemic mixture. In addition, the ligand is able to stabilize a broad variety of oxidation states on the manganese atom. Note that neither Mn(III) nor Mn(V) compounds are particularly stable without a ligand helping to stabilize them.
b. Manganese is a transition metal while magnesium is a main group metal. In case of magnesium, there are only two oxidation states known, Mg0 and Mg2+. These oxidation states are electrochemically very far away from each other in case of magnesium (E0= -2.36 V). In case of mangenese, there are many more oxidation states, which are closer together (i.e., Mn2+ --> Mn, E0= -1.18 V). Thus, it is easier for transition metal to go through a redox cycle
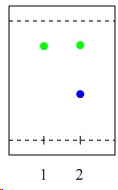
c. The active specie has a "Mn=O" function in which the Mn-atom has a formal oxidation state of +V (O=MnO2N2+). The structure of this specie is shown in the reader on page 39. The Mn-atom is located in the center of a distorted square pyramid. The basal plane is formed by the two nitrogen and the two oxygen atoms, while the oxo ligand forms the apex.
2.a. The given amount of 4-isopropylstyrene is equivalent to 4.50 mmol (=0.658 g/146.23 g/mol). He should use 0.180 mmol of the catalyst (=4.50 mmol * 0.04), which are 0.114 g (0.180 mmol*635.2 g/mol).
b. Most procedures in the literature employ dichloromethane as a solvent for the reaction because it is polar enough to dissolve the catalyst and the intermediate. Hexane or petroleum ether are not suitable because the catalyst is almost completely insoluble in these solvents. Ethyl acetate dissolves the catalyst, the alkene and the intermediates sufficiently well.
c. The reaction use a Na2HPO4/NaOH buffer, which has a pH-value of pH=11.3. The buffer is necessary to reduce the decomposition of the epoxide (acid or base catalyzed hydrolysis) and the catalyst (hydrolysis and dimerization) as well as side reactions like the chlorination of the alkene.
d.
e. Epoxides are very reactive. Even during the reaction, some of the epoxide hydrolyzes already. Once the epoxidation is completed the hydrolysis reaction is the dominant reaction in the system. In order to reduce the loss due to hydrolysis, the reaction mixture should be worked up immediately.
f. The crude product is a tan colored oil. In some cases, it might be dark brown because the catalyst was not removed properly.