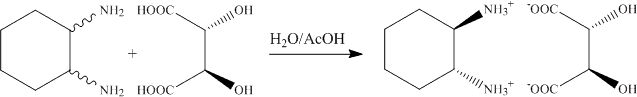
last updatedFriday, January 09, 2015
Problems Meeting #2 (Condensed Key)
1.a. The addition of the trans-1,2-diaminocyclohexane to the L-(+)-tartaric acid solution leads to the precipitation of the (R, R)-diammonium salt. The (R,R)-enantiomer is isolated because of the matched pair geometry of the cation and anion that allows for strong interactions via six hydrogen bonds to three different anions (see X-ray structure discussed in lecture).
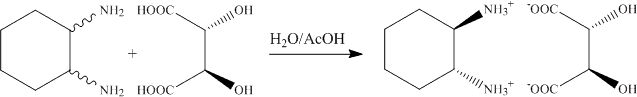
b. In the beginning of the experiment, the solution is acidic (aqueous solution of tartaric acid). Since all amine functions are fully protonated due to the low pH-value, a small amount of the target compound will precipiate at this point that dissolves as the pH-value increases.
c. In order to precipitated the salt, both amine groups are fully protonated (pKa1= ~6.5). Thus, the pH-value has to be below pH=4, which is accomplished by adding glacial acid (100 % acetic acid).
d. Based on the information given in the reader and the lecture (w/v=1:2), the student should start with 9 mL =(4.5 g*2) of solvent for the recrystallization. This given ratio is lower than the one provided in the Hanson paper (w/v=1:10) because the student sample in Chem 30CL is still wet when the student starts the recrystallization.
e. Based on the quantities used in the reader, about 6.60 g of the tartrate salt should be isolated. The diamine (50.0 mmol =(6.0 mL*0.951 g/mL)/114.2 g/mol) is the limiting reagent here (tartaric acid: 3.75 g/150.09 g/mol= 25.0 mmol). One has to keep in mind that only 50 % of the total amount of amine are used here (=25.0 mmol). If the student isolated 3.60 g (=13.6 mmol= 3.60 g/264.2 g/mol)), the yield would be 54.5 % (=13.6 mmol/25.0 mmol *100 %) in terms of the (R,R)-enantiomer. Alternatively, he could also state that he isolated 27.3 % based on the total amount of diamine added. In either case, the yield falls below the range reported by the literature (60-70 % of the available enantiomer after recrystallization).
f. The tartrate salt is reacted with sodium hydroxide solution to generate the free diamine, which is extracted into ethyl acetate. After drying the organic layer over potassium carbonate, a sample (~1.5 mL) of the solution is submitted for GC/MS analysis.
g. According to the lecture, the (S,S) enantiomer elutes before the (R,R)-enantiomer. The optical purity is 97.6 % (=1,200,000/(1,200,000+30000)*100 %), the (R,R)-enantiomer being the major isomer isolated.
2. a.The chemist's interest in chiral compounds stems from the fact that many biological systems contain chiral compounds (i.e., amino acids, sugars, terpenes, etc.). Nature seems to prefer one enantiomer over the other in most cases. In many cases, only one enantiomer of a drug displays the desired activity (eutomer) while the other one is inactive, or worse, even dangerous (distomer).
b. The stereoselectivity of a reaction fundamentally depends on the differences in activation energy (DG‡) of alternate pathways. The larger this difference is (i.e., induced by steric hindrance), the more the rates of the reactions differ and the higher the stereoselectivity of the reaction is. For a given difference in activation energies, the stereoselectivity increases as the temperature decreases.
c. The key intermediate in the Sharpless epoxidation is a dimeric Ti-specie (shown in the reader and the lecture). The alkene has to be tied to the reaction center. The alcohol reacts with the titanium and forms a covalent bond. Now, the alkene function and the hydroperoxide, which also coordinates to the Ti-atom, are is close proximity.
d. Asymmetrically substituted sulfoxides are chiral because they possess a pseudo-tetrahedral geometry: two different R-groups, one oxygen atom and a lone pair. The inversion barrier for these compounds are often very high (i.e., (CH3)PhS=O: 173 kJ/mol).