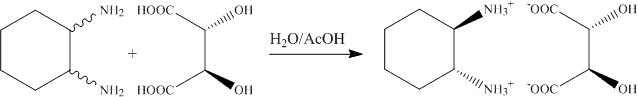
last updatedTuesday, September 29, 2015
Problems Meeting #2 (Condensed Key)
1.a. The addition of the trans-1,2-diaminocyclohexane to the L-(+)-tartaric acid solution leads to the precipitation of the (R, R)-diammonium salt. The (R,R)-enantiomer is isolated because of the matched pair geometry of the cation and anion that allows for strong interactions via six hydrogen bonds to three different anions (see X-ray structure discussed in lecture).
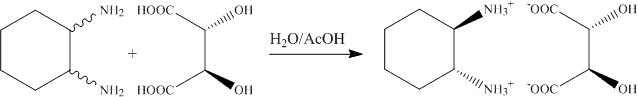
b. In the beginning of the experiment, the solution is acidic (concentrated aqueous solution of tartaric acid). Since all amine functions are fully protonated due to the low pH-value, a small amount of the target compound will precipiate at this point that dissolves as the pH-value increases.
c. In order to precipitated the salt, both amine groups have to be fully protonated (pKa1= ~6.5). Thus, the pH-value has to be below pH=4, which is accomplished by adding glacial acid (100 % acetic acid).
d. Based on the information given in the reader and the lecture (w/v=1:2), the student should start with 10.6 mL =(5.3 g*2) of solvent for the recrystallization. This given ratio is lower than the one provided in the Hanson paper (w/v=1:10) because the student sample in Chem 30CL is still wet when the student starts the recrystallization.
e. The recrystallized product is washed with water and methanol. After sucking air through the solid, the salt is placed on a watch glass or in an open large beaker to allow the sample to air dry.
f. Based on the quantities used in the reader, about 6.60 g of the tartrate salt should be isolated. The diamine (50.0 mmol =(6.0 mL*0.951 g/mL)/114.2 g/mol) is the limiting reagent here (tartaric acid: 3.75 g/150.09 g/mol= 25.0 mmol). One has to keep in mind that only 50 % of the total amount of amine are used here (=25.0 mmol). If the student isolated 3.40 g (=12.9 mmol= 3.40 g/264.2 g/mol)), the yield would be 51.5 % (=12.9 mmol/25.0 mmol *100 %) in terms of the (R,R)-enantiomer. Alternatively, he could also state that he isolated 25.7 % based on the total amount of diamine added. In either case, the yield falls below the range reported by the literature (60-70 % of the available enantiomer after recrystallization).
g. The tartrate salt is reacted with dilute sodium hydroxide solution to generate the free diamine, which is extracted into ethyl acetate. After drying the organic layer over potassium carbonate, a sample (~1.5 mL) of the solution is submitted for GC/MS analysis. Note that the solution is not concentrated or diluted.
h. According to the lecture, the (S,S) enantiomer elutes before the (R,R)-enantiomer. The optical purity is 93.5 % (=(1,500,000-50,000)/(1,500,000+50000)*100 %), the (R,R)-enantiomer being the major isomer isolated.
2. a.The chemist's interest in chiral compounds stems from the fact that many biological systems contain chiral compounds (i.e., amino acids, sugars, terpenes, etc.). Nature seems to prefer one enantiomer over the other in most cases. In many cases, only one enantiomer of a drug displays the desired activity (eutomer) while the other one is inactive, or worse, even dangerous (distomer).
b. The stereoselectivity of a reaction fundamentally depends on the differences in activation energy (DG‡) of alternate pathways. The larger this difference is (i.e., induced by steric hindrance), the more the rates of the reactions differ and the higher the stereoselectivity of the reaction is. For a given difference in activation energies, the stereoselectivity increases as the temperature decreases.
c. The key intermediate in the reduction of ketones using BINAL-H is a six-membered ring in which the aromatic ring usually assumes equatorial position to minimize the 1,3-diaxial interaction. However, if the other R-group increases in bulkiness, the equatorial position of the ring becomes less favorable. Thus, the enantioselectivity is lower for isopropylphenylketone compared to acetophenone.
d. Asymmetrically substituted phosphines are chiral because they possess a pseudo-tetrahedral geometry: three different R-groups and a lone pair. The inversion barrier for phosphines are significantly higher than for amines, which means that different enantiomers can be isolated for chiral phosphines.