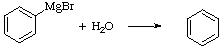 + MgBr(OH)
+ MgBr(OH)
last updatedFriday, November 07, 2014
Meeting 11 (Condensed Key)
1. a. Grignard reagents are very strong bases that react with anything that contains acidic protons i.e., water. The byproduct of the reaction, MgBr(OH), is insoluble in diethyl ether and coats the Mg-surface which prevents the formation of any new Grignard reagent.
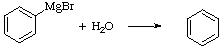 + MgBr(OH)
+ MgBr(OH)
The water is introduced in the reaction through the "wet" glassware (which is silica based and therefore polar), the reagents and the solvent, which is hygroscopic, and moisture in the air that is absorbed by the glassware and the solvent. The glassware is dried with a heat gun prior to use, the reagent bottles should be kept close and the system is only allowed to "breathe" through a drying tube.
b. The Mg-metal should consist of small, silvery shiny metal chips (turnings). Grey or black coatings indicate a more or less degree of oxidation, which is undesirable because these turnings are less reactive.
c. The addition of a few crystals of iodine causes the etching of the Mg-surface, which causes an activation of the Mg-metal.
d. The addition of the ethereal bromobenzene solution has to be carried out in a way that the reaction maintains a gentle boil. A slow addition also reduces the temperature in the reaction and suppresses the formation of biphenyl because the formation of the Grignard reagent is favored at lower temperatures. If he added the solution too rapidly, the reaction mixture would foam very heavily and turn dark brown. Ultimately, he will not isolate much benzoic acid because a lot of the Grignard reagent is consumed otherwise.
e. A large amount of the carbon dioxide will evaporate during this step causing the mixture to foam heavily due to the higher temperature of the solution and the exothermic nature of the reaction. The use of a large beaker (400 mL) is imperative to minimize the loss due to spillage. Dry-ice (=solid CO2) is the electrophile in this reaction. Its reaction with the Grignard reagent yields the benzoate.
PhMgBr + CO2 ----- > PhCOO- MgBr+
f. The dry ice should be weight right before it is needed for the reaction to reduce evaporation of the dry ice and condensation of moisture onthe dry ice. The dry ice should be transferred with a scoop or other tool and not be touched directly.
g. The addition of concentrated sulfuric acid converts the carboxylate to benzoic acid. The addition of chipped ice (=solid H2O) aim to control the temperature and to prevent the evaporating of the diethyl ether. After the ice melted, it will also serve as solvent for the Mg-salts.
PhCOO- MgBr+ + H+ ----- > PhCOOH + Mg2+ + Br-
h. The treatment of the combined organic layers with sodium hydroxide causes a deprotonation of benzoic acid and its transfer into the aqueous layer as benzoate. The unreacted bromobenzene, the biphenyl and most other organic compounds that are not acidic remain in the organic layer.
PhCOOH + OH- ----> PhCOO- + H2O
i. The next reaction is an Fischer esterification, which displays a low equilibrium constant. Since water is one of the products, any water in the setup will reduce the yield of the ester.