1. Clean up your desk and the laboratory (mandatory). Make sure that you label all your products and turn them in to the TA for proper waste management.
2. TA evaluation (appreciated)
3. Web NMR assignment (mandatory)
Part I: TLC of the crude and final product
Since there is no solvent given in the procedure, you will have to figure one out for yourself. The lab support will provide several solvents for the task: hexane, toluene, dichloromethane, methanol. You will have to place four spots on your TLC plate: benzoin, the reference compound, the crude and the purified product. Ideally, the Rf-values should vary by 0.3-0.4. This might be accomplished using one solvent or a solvent mixture.
Part II: NMR simulations
The assignment is done during the second half of the lab period in the SLC.
Using ACD labs Software, simulate and analyze som eof the the spectra for the following compounds.
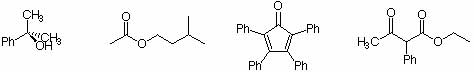
Generating the molecules
In order to draw these compounds, you will have to use ChemSketch (one of the modules of the software). Try to make use of the existing templates available in the program e.g. benzene, cyclopentane, etc. Otherwise, the drawing process is similar to the one in Spartan.
To expand an area, push both mouse buttons. A crosshair appears on the screen and a second screen on the top. Define the left edge by moving the cursor to the left side and clicking the left mouse button. Then hold the left mouse button and drag the cursor to the right and click again. A dark gray field will move with the cursor to highlight the area to be expanded. Alternately, you can also drag the markers on the top window to define the range.
1H-NMR spectra:
1. After you draw the molecule, generate the proton spectrum (ACD/labs 1H-NMR Spectrum Generator) of the compound (the default frequency should be 400 MHz at this point). Analyze the splitting pattern of the spectra and compare them with what you would expect to see. Compare the different substitution patterns of the molecules in group 2 in the 1H-NMR spectrum.
2. Compare the spectra of isopentylacetate with the one given in the reader.
3. Next, change the Default Basic Frequency (Options Menu) to 60 MHz and Recalculate the spectrum. What changes? How can you explain the change?
4. Activate the Integration feature in the Tools-menu. Can you account for its shape and the steps observed?
5. Measure the coupling constants in m-chloroethylbenzene. Can you explain the multiplet patterns?
13C-NMR spectra:
1. You can similate the 13C-NMR spectrum in the fashion (ACD/labs CNMR Spectrum Generator). Compare the different substitution patterns in the 13C-NMR spectrum of the molecules in group 2. (All generated spectra are proton-decoupled.)
2. If you check the "Off-Resonance" feature (in the Tools-Menu), the decoupling feature is deactivated. How do you the spectra change? Which conclusion can you make from this type of spectra?
3. Prepare a solution of 100 mg of isopentylacetate in 1 mL of CDCl3. How does the spectrum look like? Then change the concentration to 50 mg/mL CDCl3 and 200 mg /mL CDCl3. What changes?
4. Compare the spectra of isopentylacetate and tetraphenylcyclopentadienone with the ones given in the reader.
Part III: Web spectra
Go to the Science learning center to do the NMR and IR problems. Go to http://www.chem.ucla.edu/~webspectra/ and do the following problems
Beginner: 1, 5, 14, 19, 22, 23, 24
Intermediate 2, 15
Intermediate 12 and 13
4. YOU MADE IT......and now to a completely different topic.....Chemical animations
Although there is nothing to turn in, you should use this opportunity to sharpen you NMR analysis skills. Good luck!
Hints for the exam:
1. Be prepared for the exam. Look at the old exams posted online or in the exam reader, and try to solve them on your own. If you have questions, please contact your TA or instructor. As you will notice, the exams are relatively long, not everybody will be able to finish them. Make sure that you give short answers and don't write long essays. Quality over quantity!!!
2. There will be no tables provided for IR and NMR spectroscopy. You will need to memorize some basic numbers.
3.The exam will take place on August 3, 2004 at 11:00 - 1:00 pm in Math4000A. You will only be allowed to use a pen, a ruler and a non-graphing calculator during the exam. It is your responsibility to bring these items with you to the exam. The review will start on July 29, 2004 at 11 pm. The rest will be covered on August 2, 2004 from 4-6 pm (Boelter 5249)
4. DON'T FORGET TO TURN IN YOUR LAB NOTEBOOK ON THE DAY OF THE EXAM!