NMR simulations (has to be done in the SLC since the Chemdraw and ACD program is not available elsewhere on campus!)
The purpose of this assignment is to analyze the splitting patterns of various substitution patterns and to observe the changes in spectra when the structure changes. The assignment is mandatory and is done during the second half of the lab period in SLC. The assignment is due during your in-lab meeting in the week 10 (credit: 10 points). Using Chemdraw and the ACD labs Software, simulate and analyze some of the the spectra for the following compounds.
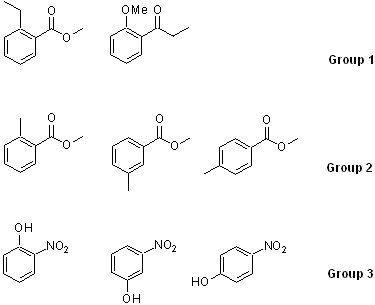
Generating the molecules
In order to draw these compounds, you will have to use Chemdraw. Try to make use of the existing templates available in the program i.e. benzene, etc. Otherwise, the drawing process is similar to the one in Spartan. However, you cannot draw the molecule in Spartan and then copy it over because the two programs use different protocols!
To expand an area, click both mouse buttons. A crosshair appears on the screen and a second screen on the top. Define the left edge by moving the cursor to the left side and clicking the left mouse button. Then hold the left mouse button and drag the cursor to the right and click again. A dark gray field will move with the cursor to highlight the area to be expanded. Alternatively, you can also drag the markers on the top window to define the range.
1H-NMR spectra:
1. After you draw the molecules, highlight the molecule and go to "Extensions - Run Programs" to start the ACD module. Pick the H-NMR feature to simulate the H-NMR spectrum . Set the default frequency to 400 MHz in the beginning. (Options-Default Basic Frequency-400).
a. For group 1, analyze the peak locations and the splitting patterns for the aliphatic range.
b. For group 2, analyze the splitting pattern of the aromatic range and rationalize the location for the Me-signal on the ring. Compare the obtained spectra with the literature spectra that can be found on www.sigmaaldrich.com or sdbs.
c. For group 3, analyze the splitting pattern of the aromatic range and rationalize the locations for the OH signal in each spectrum.
2. Next, change the Default Basic Frequency to 60 MHz and recalculate the spectra for group 2. What changes? How can you explain the change?
3. Using the molecules of group 2, activate the Integration feature in the Tools-menu. Can you account for its shape and the steps observed?
13C-NMR spectra:
1. Highlight the molecule and go to "Extensions - Run Programs" to start the ACD module. Pick the C-NMR feature to simulate the C-NMR spectrum .You can simulate the 13C-NMR spectrum in the fashion (ACD/labs CNMR Spectrum Generator). Compare the different substitution patterns in the 13C-NMR spectrum of the molecules in group 2. (All generated spectra are proton-decoupled.)
2. If you check the "Off-Resonance" feature (in the Tools-Menu), the decoupling feature is deactivated. How do the spectra change? Which conclusion can you draw from this type of spectra?
3. The program also allows you to do a "virtual lab". In the C-NMR module, go to the Show-Solvent lines item. A window pops up that allows you to change the solvent, its quantity and the sample weight.
a. "Prepare" a solution of 10 mg of o-nitrophenol in 1 mL of CDCl3. How does the spectrum look like?
b. Then change the concentration to 100 mg sample and 1 mL C6D6. Which changes do you observe in the spectrum compared to a.? Rationalize these observations.