Week 8 Problem Set 30BL
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up
1. Why does your glassware to be dry in this experiment? Explain briefly.
2. Many Grignard reactions employ the use of an alkyl bromide as a starting material. Explain briefly why.
3. The following three solvents are available for a Grignard reaction: diethyl ether, dichloromethane and toluene Which one would you use? Rationalize your choice.
4. Why is 10% sulfuric acid added after the reaction is cooled?
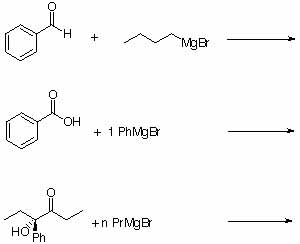
5. Predict the products for the following reactions. Include the approximate ratio if more than one product obtained and the correct stereochemistry.

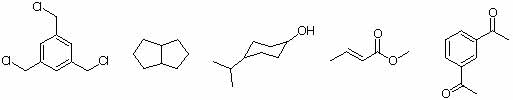
6. How many signals do you expect to observe in the 13C{1H}-NMR spectra of the following compounds? In which of the following ranges do those signals show up: 0-50 ppm, 50-100 ppm, 100-160 ppm, 160-220 ppm
Note: I will be available for questions in my office on Monday (Veteran's Day).