Problems Set - Meeting #7
1. Most Diels-Alder reactions are exothermic? Explain briefly.
2. What drives this Diels-Alder reaction performed in the laboratory?
3. A student dissolves 0.60 g of tetraphenylcyclopentadienone and 0.33 g of anthranilic acid in 5 mL of tetrahydrofuran and then adds 0.6 mL of isopentyl nitrite.
a. How much carbon monoxide (in units of mL) is given off (assume reaction goes to completion)?
b. Which size of flask would you use to do the reaction?
c. What do you observe during the reaction?
4. What does the term "in-situ" mean? When do people use this technique?
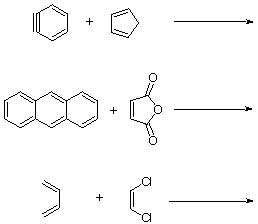
5. Predict the major products for the following reactions. Include the correct stereochemistry.

6. Why does the dienophile have to be in c-cis configuration for the Diels-Alder reaction to occur? Explain briefly.
7. A student performs the following sequence of experiments:
A--> B --> C --> D
In the first reaction, he isolates the product in 85% yield. The second reaction runs in 60% yield and the third in 70% yield. If he started off with 0.6 mol of A, how many moles of D did he isolate assuming he used up all of his intermediates?