stationary phase:
silica
mobile phase:
hexane
General Announcement (Please read!)
It has come to my attention that some students are not clear about certain policies in this course. There is no such policy in this lab course which discourages students from asking questions. The reason why you are here is to learn. This should also result in questions from the the student's side, which are welcome by the teaching assistants and the instructor. However, it is expected that the student comes prepared to the lab section in order to be able to complete the experiment in a timely fashion. The majority of the questions regarding the experiment (theory and practical issues) can be answered during lecture and office hours. The question should demonstrate the student's ability to analyze the problem and be as specific as possible.
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. Referring to the formation of the enolate, answer the following questions.
a. Why are the methylene protons in dibenzyl ketone more acidic than the ones in 1,3-diphenyl propane?
b. Why is 95% ethanol a poor choice as solvent in this week's reaction?
2. What drives the reaction towards the formation of the tetraphenylcyclopentadienone?
3. A student isolated 1.5 g of a crude product. In order to purify it, he has three solvents accessible: methanol, ethyl acetate, pentane
| Solvent | Temperature in oC | Solubility in g/100mL |
| Methanol | 0 | 2.0 |
| 20 | 2.1 | |
| 40 | 2.3 | |
| 60 | 2.7 | |
| Ethyl acetate | 0 | 0.1 |
| 20 | 0.2 | |
| 40 | 0.4 | |
| 60 | 0.8 | |
| 80 | 1.5 | |
| Pentane | 0 | 0.05 |
| 20 | 0.1 | |
| 40 | 0.2 | |
a. Which solvent should the student choose for recrystallization? Rationalize your choice.
b. How much of the purified compound (in g and %) would the student recover if she performs the recrystallization correctly?
c. Which solvent should she choose if she want to run a reaction that has this compound as product?
d. Why are there no solubility values given for 60 oC and 80 oC for pentane?
3. The final product is recrystallized from a mixture of 95% ethanol and toluene (1:1).
a. Why do we use a solvent mixture here?
b. What would happen if only toluene is used for the recrystallization?
c. What would happen if the mixture is boiled for an extended time?
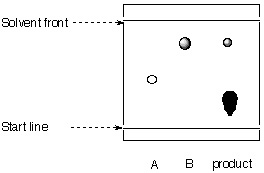
4. The researcher is given the following TLC from his assistant.
 |
stationary phase: mobile phase: |
a. Calculate the Rf-values for all spots.
b. What can be said about the purity of the progress of the reaction?
c. Is the chromatogram obtained correctly?
5. A student plans to obtain the UV spectrum for tetraphenylcyclopentadienone. He prepares a solution by dissolving some crystals in 10 mL of solvent. The solution is dark purple in color. He observes the following UV-Vis spectrum for his solution.
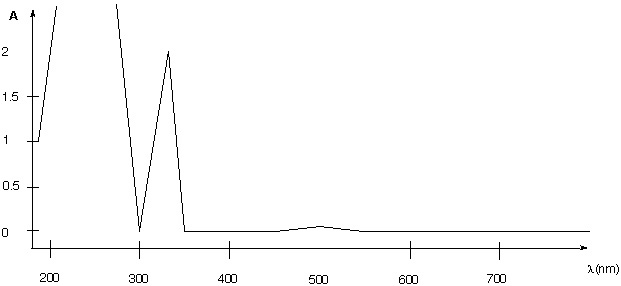
a. Determine the molar extinction coefficients.
b. The spectrum acquired incorrectly. What has to be changed and why?