stationary phase:
silica-C18
mobile phase:
methanol
Meeting 6 Problem Set 30BL
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. Which solvent is used in this week's reaction and why? Explain.
2. Student A isolated 10 g of his crude product. In order to purify it, he has three solvents accessible: water, methanol, tetrahydrofuran
| Solvent | Temperature in C | Solubility in g/10mL |
| Water | 0 | 0.1 |
| 20 | 0.15 | |
| 40 | 0.2 | |
| 60 | 0.25 | |
| Methanol | 0 | 0.1 |
| 20 | 0.4 | |
| 40 | 0.6 | |
| 60 | 1.0 | |
| 70 | 4.0 | |
| Tetrahydrofuran | 0 | 1.6 |
| 20 | 1.65 | |
| 40 | 1.7 | |
| 60 | 1.75 | |
| 80 | 1.8 |
a. Which solvent should she choose for recrystallization? Rationalize your choice.
b. How much of the purified compound (in g and %) would he recover if she performs the recrystallization correctly?
c. What can be said about the polarity of the compound?
3. The final product is recrystallized from a mixture of 95% ethanol and toluene (1:1). Why do we use a solvent mixture here? What would happen if only toluene is used for the recrystallization?
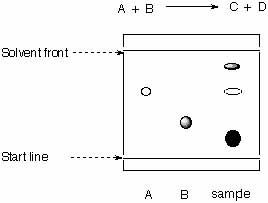
4. The researcher attempts to evaluate the progress of his reaction by TLC.
 |
stationary phase: mobile phase: |
a. Calculate the Rf-values for all spots.
b. What can be said about the progress of the reaction?
c. Is the chromatogram obtained correctly?
d. What are the relative polarities of A, B and the products?
5. Student B obtains a compound (m.w.=100 g/mol) and decides to obtain an UV spectrum for the compound. He prepares a solution by dissolving 2 mg in 10 mL of solvent and then diluting this solution 10 fold. Using a 10 mm cuvette, he observes an absorbance of A=0.15 A.U. at l=340 nm and A=1.7 A.U. at l=280 nm. Determine the molar extinction coefficients.
a. Which color does the compound have to the observer?
b. Is the spectrum correctly acquired? If not, what has to be changed?