stationary phase:
silica
mobile phase: toluene/hexane
Week 6 Problem Set 30BL
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. Which solvent is used in this week's reaction and why? Explain.
2. Student A isolated 1 g of his crude product. In order to purify it, he has three solvents accessible: methanol, hexane and ethyl acetate.
| Solvent | Temperature in C | Solubility in g/mL |
| Methanol | 0 | 0.1 |
| 20 | 0.15 | |
| 40 | 0.2 | |
| 60 | 0.25 | |
| Hexane | 0 | 0.2 |
| 20 | 0.4 | |
| 40 | 0.6 | |
| 60 | 1.0 | |
| 70 | 2.5 | |
| Ethyl acetate | 0 | 1.5 |
| 20 | 1.6 | |
| 40 | 1.7 | |
| 60 | 1.8 | |
| 80 | 1.9 |
a. Which solvent should he chose for recrystallization? Rationalize your choice.
b. How much of the purified compound (in g and %) would he recover if he performs the recrystallization correctly?
c. What can be said about the polarity of the compound?
3. The final product is recrystallized from a mixture of 95% ethanol and toluene (1:1).
a. A student boils the solution for a significant amount of time without placing a watch glass on the top. After the recrystalliztion, he isolates the product in 10% yield. Rationalize this observation.
b. What would change if the solvent mixture used is 95% ethanol and toluene (5:1)?
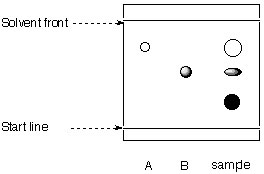
4. The researcher attempts to evaluate the purity of his product by TLC.
 |
stationary phase: mobile phase: toluene/hexane |
a. Calculate the Rf-values for A, B and the spots in the product lane.
b. What can be said about the progress of the reaction?
c. Is the chromatogram obtained correctly?
d. What are the relative polarities of A, B and the product?
5. Student B obtains a yellow compound and decides to obtain an UV spectrum for the compound. From the literature, she found out that the compound is supposed to absorb at a wavelength of l1= 330 nm and l2=450 nm. The given molar extinction coefficients are e1=5000 L*(mol*cm)-1 and e2=350 L*(mol*cm)-1.
a. Which concentration would lead to a 'good' reading for the absorbance assuming that she uses a 10 mm cuvet?
b. Which of the peaks is responsible for the yellow color of the compound? Explain.
c. What is a chromophore?