stationary phase:
silica
mobile phase:
heptane
General Announcement (Please read!)
It has come to my attention that some students are not clear about certain policies in this course. There is no such policy in this lab course which discourages students from asking questions. The reason why you are here is to learn. This should also result in questions from the the student's side, which are welcome by the teaching assistants and the instructor. However, it is expected that the student comes prepared to the lab section in order to be able to complete the experiment in a timely fashion. The majority of the questions regarding the experiment (theory and practical issues) can be answered during lecture and office hours. The question should demonstrate the student's ability to analyze the problem and be as specific as possible.
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. Why is absolute ethanol used in this week's reaction? Explain briefly.
2. Student A isolated 12 g of his crude product. In order to purify it, he has three solvents accessible: water, acetone, hexane
| Solvent | Temperature in oC | Solubility in g/10mL |
| Hexane | 0 | 0.2 |
| 20 | 0.25 | |
| 40 | 0.3 | |
| 60 | 0.35 | |
| Acetone | 0 | 0.2 |
| 20 | 0.4 | |
| 40 | 0.8 | |
| 60 | 1.6 | |
| Water | 0 | 1.6 |
| 20 | 1.65 | |
| 40 | 1.7 | |
| 60 | 1.75 | |
| 80 | 1.8 |
a. Which solvent should the student choose for recrystallization? Rationalize your choice.
b. How much of the purified compound (in g and %) would the student recover if she performs the recrystallization correctly?
c. What can be said about the polarity of the compound?
3. The final product is recrystallized from a mixture of 95% ethanol and toluene (1:1).
a. Why do we use a solvent mixture here?
b. What would happen if only toluene is used for the recrystallization?
c. What would happen if the mixture is boiled for an extended time?
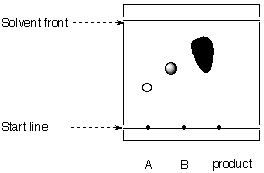
4. The researcher is given the following TLC from his assistant.
 |
stationary phase: mobile phase: |
a. Calculate the Rf-values for all spots.
b. What can be said about the purity of the product?
c. Is the chromatogram obtained correctly?
5. Student B obtains a compound (m.w.=250 g/mol) and decides to obtain an UV spectrum for the compound. He prepares a solution by dissolving 5 mg in 25 mL of solvent and then diluting this solution 10 fold. Using a 10 mm cuvette, he observes an absorbance of A=0.25 A.U. at l=330 nm and A=1.5 A.U. at l=250 nm.
a. Determine the molar extinction coefficients.
b. Which color does the compound have to the observer?
c. The spectrum acquired incorrectly. What has to be changed and why?