Week 6 Problem Set 30 BL
Useful Link: Refractometer
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
This Week's Experiment: Esterification Reaction
1. What is the role of the sulfuric acid in this week's experiment? How about nitric acid? Explain.
2. Explain how the workup serves to isolate the ester. Supply a flow chart indicating in which layer the ester can be found at each step. (Hint: What organic compounds are present after the reflux?)
3. A student observes a refractive index of nD16=1.3437. What is the refractive index at 20 C? Which information does he get from the refractive index?
4. How does the yield (in g) for the esterification change if the student
a. adds only 10% of the sulfuric acid asked for in the procedure and runs the reaction for the same amount of time
b. adds twice as much acetic acid in her reaction
c. refluxes the mixture for five days instead of 60 minutes
d. adds 20% less isoamyl alcohol
e. does not add the sulfuric acid at all
f. runs the reaction at room temperature
g. uses sodium hydroxide for the work-ip to remove the acid
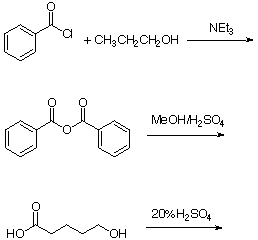
5. Predict the major product of following reactions.
a.

b. Why does the last reaction require only 20% sulfuric acid?
6. Do questions 4, 7, 8 and 11 on p. 140 in Landgrebe.