Week 5 Problem Set 30 BL (Esterification Reaction)
Useful Link: Refractometer
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. What is the role of the sulfuric acid in this week's experiment? Can it be replaced by hydrochloric acid? Explain.
2. Explain how the workup serves to isolate the ester. Supply a flow chart indicating in which layer the ester can be found at each step. (Hint: What organic compounds are present after the reflux?)
3. A student observes a refractive index of nD18=1.3532. What is the refractive index at 20 C? Which information does he get from the refractive index?
4. How does the yield (in g) for the esterification (of acetic acid with isoamyl alcohol) change if the student
a. adds 10% of the sulfuric acid and runs the reaction for the same amount of time
b. adds 10% more acetic acid in her reaction
c. refluxes the mixture for a days instead of 60 minutes
d. adds 20% less isoamyl alcohol
e. adds 50% sulfuric acid
f. runs the reaction at room temperature
g. uses sodium hydroxide for the work-up
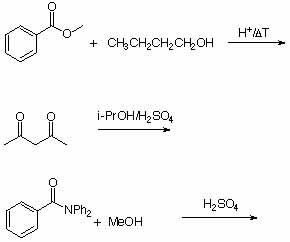
5. Predict the major product of following reactions.

6. Do questions 4, 7, 8 and 11 on p. 140 in Landgrebe.
7. Why do we reflux the mixture for one hour?