General Announcement (Please read!)
1. It has come to my attention that some students are not clear about certain policies in this course. There is no such policy in this lab course which discourages students from asking questions. The reason why you are here is to learn. This should also result in questions from the the student's side, which are welcome by the teaching assistants and the instructor. However, it is expected that the student comes prepared to the lab section in order to be able to complete the experiment in a timely fashion. The majority of the questions regarding the experiment (theory and practical issues) can be answered during lecture and office hours. A question also should demonstrate the student's ability to analyze the problem and be as specific as possible in his/her inquiry. A simple comment like "I don't understand this" is not particularly helpful for the person that you are asking since they do not know which part you are having problems with.
2. In the future, please do not study outside my office (YH3077E). My colleagues in the office suite try to get some work done, and the noise outside is rather disturbing i.e. cell phones, etc. The tables in the office suite are reserves for office hours, etc.
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. Referring to the Aldol reaction carried out in the lab, answer the following questions. Show pertinent equations where appropriate.
a. Why is it imperative to use absolute ethanol as a solvent in the reaction?
b. One of the driving forces in the reaction is the intramolecular last step. What does this mean and why does this favor this step?
c. A student obtained 0.265 g (dry weight) of the crude product. How much solvent should he use to recrystallize the crude product?
d. Why is extensive boiling during the recrystallization step unwise?
e. Why is the use of a boiling stick preferred over a boiling stones?
f. Why is it important to allow the solution to cool down slowly during the recrystallization step?
2. A student isolates 2.00 g of a crude product Z that shows the following solubilities (in g/10 mL) in absolute ethanol, dichloromethane and hexane. The impurity to be removed is weakly polar.
| Temp (oC) |
EtOH
|
DCM | hexane |
| 0 | 0.1 | 1 | 5 |
| 20 | 0.2 | 3 | 7 |
| 40 | 0.3 | 5 | 9 |
| 60 | 0.4 | XXX | 11 |
| 80 | 0.5 | XXX | XXX |
| 100 | XXX | XXX | XXX |
a. Which solvent should the student use for recrystallization? Rationalize your choice.
b. Assuming the recrystallization is carried out correctly, how much of the pure product would be recovered? Show your work.
c. What happens to the boiling point of the solvent if the amount of solute increases? Explain.
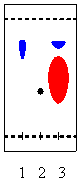
3. While running the following reaction
A + B --> 2 C
a student acquires a TLC for his reaction mixture using a plate coated with silica and a mixture of diethyl ether and hexane (1:2) as eluent. He observes the following result

Note: Lane 1 is compound A, lane 2 is compound B and lane 3 is the sample from the reaction mixture.
a. Determine the Rf-values for all compounds.
b. What can be said about the progress of the reaction?
c. His neighbor decides to use a solvent mixture dichloromethane as eluent instead. Which changes would he observe?
d. Why is one of the spots in lane 3 so large? Which problem does this pose here? How can the student fix the problem?