Week 5 Problem Set 30 BL (Esterification Reaction)
Useful Link: Refractometer
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. Extraction with sodium bicarbonate
a. Could the extraction be performed with NaOH solution?
b. Why do you have to be careful in the beginning? Show pertinent equations for the extraction with NaHCO3?
c. Why is sodium bicarbonate considered a weak base?
2. Refractive index of isoamyl acetate
a. A student obtains a refractive index of 1.4021 at 24 oC. What would be the refractive index of his compound at 15 oC?
b. What can be said about the purity of his sample?
3. A student refluxes a mixture 20.0 g of benzoic acid, 5 mL of methanol and 1 mL of concentrated sulfuric acid for two hours. After the work-up, the student isolated 14 g of the ester.
a. Suggest a procedure to isolate the ester.
b. Determine the yield of the reaction. Show all calculations.
c. How could the student improve the percentage yield of this reaction?
d. What would happen if he forgets the add the sulfuric acid?
e. Why does she have to reflux the reaction mixture?
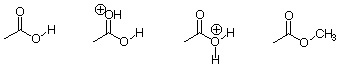
4. Perform an AM1 calculation for following molecules. Note that the second and third molecule are cations.

a. Compare the C=O and C-O in the different molecules. What can you conclude?
b. Generate the HOMO and LUMO orbitals for the first three molecules. Compare and rationalize the differences