updated last Thursday, January 25, 2007
Announcements
1. The IR assignment for this quarter was posted on Wednesday. The assignment is due on Friday February 2, 2007 at 5 pm in YH3077E. No late assignments will be accepted.
2. The next workshop (1/29/2007, 5 pm) will cover IR spectroscopy. The students will work in small groups on the practice assignment posted on the website. This exercise is meant to improve the problem solving skills.
_______________________________________________________________________________________________________________________
Week 4 Problem Set - 30 BL (Turn in your computer assignment during week 5)
- Include Part I into your prelab.
- DO PARTS II and III DURING YOUR ASSIGNED LAB PERIOD.
- ANSWERS TO THESE QUESTIONS ARE TO BE DONE IN YOUR LAB NOTEBOOK.
- This assignment is worth 10 points and should take about 45 minutes to complete.
- ATTENTION: PC_Spartan 2002 application has a problem printing sometimes!
BUT THERE IS AN ALTERNATIVE FOR PRINTING
FROM SPARTAN. Here's what do:
- Open a blank document in MS Word.
- Copy the molecule from Spartan (Ctrl C)
- Paste into Word
- And print -- voila!
Part I: Phase Transfer Oxidation (due in prelab)
1. A student acquires a GC spectrum for his final product.
Solvent: dichloromethane, Column: polyethylene glycol, 30 m, 0.25 mm, Temperature: T=120 oC (isothermal)
Results:
| Time (min) |
Height (pA) |
halfwidth (min) |
Compound |
| 1.3 |
20000 |
0.20 |
|
| 2.5 |
100 |
0.15 |
A |
| 3.5 |
900 |
0.25 |
B |
| 5.0 |
500 |
0.10 |
C |
a. Determine the composition of his final product that all three compounds have the same response factor.
b. What can be said about the polarity of the compounds?
c. How would the spectrum change if the temperature would be lowered to T=80 oC for the run?
d. How would the spectrum change if the reaction mixture would be dissolved in methanol?
e. What does "pA" stand for in the table above?
2. A separatory funnel is used this week during the work-up. Answer the following questions.
a. Which tests should be performed prior to using the separatory funnel?
b. If sodium bicarbonate is used for extraction, the separatory has to be vented frequently. Explain briefly and show pertinent equations.
c. Why should a funnel be used to transfer the solution into the separatory funnel?
3. Referring to the reaction carried out in the lab, answer the following questions.
a. (NBu4)(HSO4) is used in the reaction. What does this compound exactly do?
b. What is the function of NaOCl in the reaction?
c. Why is it important that the reaction mixture is stirred vigorously?
d. Explain briefly how the column chromatography aids the purification of the final product.
e. A student decides to use hexane instead of ethyl acetate as a solvent for the reaction. What would he observe?
PART II. (Reduction of Camphor, In-lab assignment)
This assignment is to be completed in the UCLA Science Learning Center computing labs during the lab period (or afterwards if you don't complete it in the allotted time). The assignment is due during meeting 5 and is worth 10 points.
1. Optimize the above structural geometry of borneol, camphor and isoborneol. (see below instructions). Compare the heat of formation of borneol and isoborneol. Which one is thermodynamically more stable? How can you rationalize the differences?
2. Determine the dipol moment for borneol, camphor and isoborneol. (see below instructions). In the GC spectrum, camphor has the shortest retention time, then isoborneol and borneol last. Can you rationalize the GC results based on the calculated dipole moments of these compounds? (Note: The GC column is relatively non-polar.) Do not apply any constraints in this part!
Instructions:
- Build, minimize and save the structure. (don't forget to Minimize the structure after building it!!)
 this is the minimize button. Always minimize your structure before leaving the building mode.
this is the minimize button. Always minimize your structure before leaving the building mode.
- click on
 to enter View Mode
to enter View Mode
- Select Calculations from the Setup menu.
The following window should appear. Select the options shown.
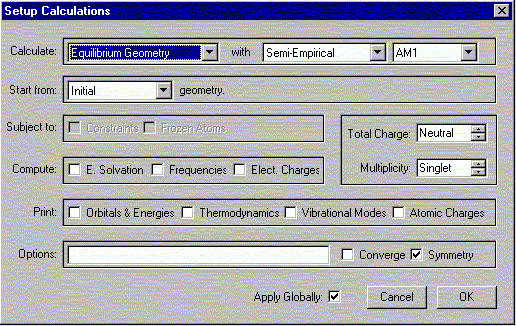
Verify the Charge is Neutral and Multiplicity is Singlet. Click OK.
- Select Submit from Setup menu.

- When the calculation is completed you will be notified.
- Select Output from the Display menu.
Record the heat of formation value given
See these Helpful Hints for manipulating structures!
Note:
| 1 au = 1 hartree = 627.5 kcal/mol |
| 1 eV = 23.06 kcal/mol |
| 1 hartree = 27.21 eV |
| 1 Ångstrom = 1.889762 atomic units = 10-8 cm |
PART III. (Aldol Condensation, In-lab assignment)
|
1. Calculate the dipole moment for dihedral angles:
0 through 180 degrees in 15 degree increments.
Plot the dipole moment of benzil vs. the dihedral angle.
|
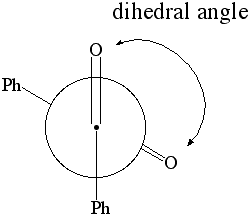
Newman Projection of Benzil
|
Instructions:
- click on Constrain Dihedral angle tool,

- select the atoms shown below in the specific sequence O..C..C..O. This will define two planes O1C1C2 and C1C2O2, which are going to be rotated towards each other in this part.
- Click on the lock tool,
 , in the bottom right corner so that it looks like this,
, in the bottom right corner so that it looks like this, 
- Set the dihedral angle to 0 and hit the ENTER key.
- click on the minimize tool,
 , and wait for the operation to complete.
, and wait for the operation to complete.
- click on the minimize tool,
 , a second time and wait for the operation to complete.
, a second time and wait for the operation to complete.
- Select Calculations from the Setup menu. The following window should appear. Select the options shown.

- Make sure the
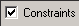
- Click OK to close the window.
- Select Submit from Setup menu. When the calculation is completed you will be notified.
- Under the Display menu, select Properties.

- A window should appear (the values in this example should differ from yours). If there are no values in the window then you need to double click on the molecule.

- Record the dipole moment and energy given. (In this example the dipole = 3.9 debye).
- Calculate the dipole moment for dihedral angles: 0 through 180 degrees in 15 degree increments. (Hint: Just click on the pink bond and change the value in the box.). Do not define a new constraint at this point. This will confuse the program completely!
- For the higher angles (>120 degrees), you might have to uncheck the symmetry box in the setup calculation menu. However, sometimes it works better if the approach is for the higher angles is from 180 degrees going down.
- Using EXCEL, plot the dipole moment and energy of benzil vs. the dihedral angle (use MS Excel, XY Scatter plot). Rationalize the observed trends.
 this is the minimize button. Always minimize your structure before leaving the building mode.
this is the minimize button. Always minimize your structure before leaving the building mode. to enter View Mode
to enter View Mode






