updated last Tuesday, April 13, 2010
Announcements
1. IR assignment
The IR assignment for this quarter is due by Friday, April 16, 2010 at 5 pm in YH 3077E. No late assignments will be accepted. If you are not at school on Friday, you are welcome to drop off the assignment earlier (Mailbox outside the instructor's office or instrutor directly). Do not slide it through under the instructor's office door. Do not drop it off in the faculty mailbox either.
2. Safety
a. Make sure to be careful when you handle glassware. This applies to the use during a reaction as well as the cleaning part. Broken glassware can cause very deep cuts which have often to be stitched up. If chemicals get into the open wound, the wound have to be specially treated and the healing process takes often more time as well.
b. It has come to the attention of the instructor that students have been dumping acetone down the sink. This is entirely unacceptable. If a student will be caught doing this, s/he will receive an automatic zero for the entire lab meeting. Repeat offenders will be dismissed from the course due to safety issues. CAL OSHA will spell out very heafty fine for such violations!
c. The students are not allowed to use any reader in the lab. All information that is needed to carry out the experiment has to be included into the pre-lab. A student that uses the readers in the lab (for whatever reason) is deemed to be unfit for the lab and will receive a zero -n-lab evaluation score for this lab meeting.
d. Make sure to inform yourself about the hazards of the chemicals that you are using in the lab. It is your responsibilty to be informed about that for your own protection. Please keep in mind that you can also be tested in this knowledge.
3. Work shop
The next workshops will take place on April 19, 2010 at 5-5:50 pm in CS 24. It will cover NMR spectroscopy.
_______________________________________________________________________________________________________________________
Week 4 Problem Set - 30 BL (Turn in your computer assignment during meeting 5)
- Include Part I into your prelab.
- DO PARTS II and III DURING YOUR ASSIGNED LAB PERIOD WHILE THE REACTION IS STIRRING.
- ANSWERS TO ALL OF THESE QUESTIONS SHOULD BE IN YOUR LAB NOTEBOOK.
- This assignment is worth 10 points and should take about 45 minutes to complete. If you do not complete it during tihs time, you will have to come back later. This part is due at the beginning of meeting 5.
Part I: Phase Transfer Oxidation (due in prelab)
1. Please watch the following video (MP4-format) and read the appropriate chapter in the "Survival Kit Reader". Then take the quiz below.
Video: Extraction
Online Quiz: http://bacher.chem.ucla.edu/TakeQuiz/?id=c51ce410c124a10e0db5e4b97fc2af39
In order to take the quiz, you have to go through a UCLA ICP address. This means that you either have to use your Bruin-Online account or go through the VPN (Vitual Private Network, software can be found here: http://www.bol.ucla.edu/services/vpn/) to have this UCLA ICP address.
To log in, use your last name and your student ID. If you are experiencing problems, contact the instructor via email and include your full name (indicated which one is your last name), your student ID, section and TA. (Hint: Think very careful about each response since many of the questions have more than one answer to them! Many students come up with the most obvious one and miss some of the details which leads to a zero score for the question!) Even though you can take the quiz until one hour prior to meeting 2 of your section, you should not delay taking it since there might be some problems with the server or the login. Also, there seem to be problems with MAC systems, Safari and Google Chrome Browser. The best is using IE 7.0 or Firefox. After you submit the answers, your score has to appear on your screen. If this does not happen, you will have to retake the quiz. (There will not be any possibility to retake the quiz weeks later since you are supposed to show preparedness at the point in time when you enter the lab!). The quiz is worth 10 points.
2. Referring to the phase transfer reaction carried out in the lab, answer the following questions.
a. In which way does TBHS promote the reaction? Would the reaction also take place if the student forgets to add this compound in his reaction?
b. What is used as oxidant in this reaction? Rationalize the choice.
c. Why is it important that the reaction mixture is stirred vigorously?
d. Which observation will the student make if the reaction proceeds according to plan?
e. Once the reaction is completed, the two layers are separated. The organic layer is subsequently extracted with water. Which purpose does this step serve?
f. Afterwards, the organic layer is dried over anhydrous magnesium sulfate. How does the student know that he added an adequate amount of drying agent?
g. After the drying agent has been removed, the majority of the solvent is evaporated. Why is this necessary?
h. Why is it important to use a very small piece of cotton in the tip of the pipette for the column chromatography step?
PART II. (Reduction of Camphor, In-lab assignment)
This assignment is to be completed in the UCLA Science Learning Center computing labs during the lab period (or afterwards if you don't complete it in the allotted time). The assignment is due during meeting 5 and is worth 10 points.
Determine the dipole moment for borneol, camphor and isoborneol. (see below instructions). In the GC spectrum, camphor exhibits the shortest retention time, then isoborneol and borneol last. Can you rationalize the GC results based on the calculated dipole moments of these compounds? (Note: The GC column is relatively non-polar, HP-5) Do not apply any constraints in this part!
Instructions:
- Build, minimize and save the structure. (don't forget to Minimize the structure after building it!!)
 this is the minimize button. Always minimize your structure before leaving the building mode.
this is the minimize button. Always minimize your structure before leaving the building mode.
- click on
 to enter View Mode
to enter View Mode
- Select Calculations from the Setup menu.
The following window should appear. Select the options shown.
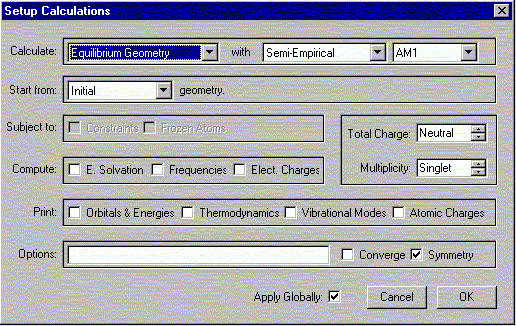
Verify the Charge is Neutral and Multiplicity is Singlet. Click OK.
- Select Submit from Setup menu.

- When the calculation is completed you will be notified.
- Select Output from the Display menu.
Record the heat of formation value given
See these Helpful Hints for manipulating structures!
PART III. (Aldol Condensation, In-lab assignment)
|
1. Calculate the dipole moment and the energy for dihedral angles:
0 through 180 degrees in 15 degree increments.
|
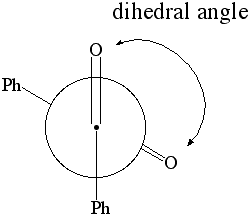
Newman Projection of Benzil
|
Instructions:
- click on Constrain Dihedral angle tool,

- select the atoms shown below in the specific sequence O..C..C..O. This will define two planes O1C1C2 and C1C2O2, which are going to be rotated towards each other in this part.
- Click on the lock tool,
 , in the bottom right corner so that it looks like this,
, in the bottom right corner so that it looks like this, 
- Set the dihedral angle to 0 and hit the ENTER key.
- click on the minimize tool,
 , and wait for the operation to complete.
, and wait for the operation to complete.
- click on the minimize tool,
 , a second time and wait for the operation to complete.
, a second time and wait for the operation to complete.
- Select Calculations from the Setup menu. The following window should appear. Select the options shown.

- Make sure the
- Click OK to close the window.
- Select Submit from Setup menu. When the calculation is completed you will be notified.
- Under the Display menu, select Properties.

- A window should appear (the values in this example should differ from yours). If there are no values in the window then you need to double click on the molecule.

- Record the dipole moment and energy given. (In this example the dipole = 3.9 debye and the energy is -0.00947 a.u.) Hint: Just click on the pink bond and change the value in the box. Do not define a new constraint at this point. This will confuse the program completely!
- For the higher angles (>120 degrees), you might have to uncheck the symmetry box in the setup calculation menu. However, sometimes it works better if the approach is for the higher angles is from 180 degrees going down. In some cases, the symmetry feature has to be removed and put back in afterwards.
- Using EXCEL, plot the dipole moment and energy of benzil vs. the dihedral angle (use MS Excel, XY Scatter plot). Rationalize the observed trends.
 this is the minimize button. Always minimize your structure before leaving the building mode.
this is the minimize button. Always minimize your structure before leaving the building mode. to enter View Mode
to enter View Mode






