updated last Monday, October 15, 2007
Announcements
1. The IR assignment for this quarter is due on Friday October 26, 2007 at 5 pm in YH3077E. No late assignments will be accepted.
2. The workshop on 10/22/2007 at 5 pm will cover NMR spectroscopy I. _______________________________________________________________________________________________________________________
Week 4 Problem Set - 30 BL (Turn in your computer assignment during week 5)
- Include Part I into your prelab.
- DO PARTS II and III DURING YOUR ASSIGNED LAB PERIOD.
- ANSWERS TO THESE QUESTIONS ARE TO BE DONE IN YOUR LAB NOTEBOOK.
- This assignment is worth 10 points and should take about 45 minutes to complete.
- ATTENTION: PC_Spartan 2002 application has a problem printing sometimes!
BUT THERE IS AN ALTERNATIVE FOR PRINTING
FROM SPARTAN. Here's what do:
- Open a blank document in MS Word.
- Copy the molecule from Spartan (Ctrl C)
- Paste into Word
- And print -- voila!
Part I: Phase Transfer Oxidation (due in prelab)
1. A student acquires a GC spectrum for his final product from the reduction reaction.
Solvent: dichloromethane, Column: HP-5, l=30 m, 0.25 mm, Temperature: T(initial)=100 oC, T(final)=180 oC, t = 8 min
Results:
| Time (min) |
Height (pA) |
halfwidth (min) |
Peak |
| 1.0 |
10000 |
0.20 |
A |
| 2.5 |
120 |
0.15 |
B |
| 2.6 |
1200 |
0.25 |
C |
| 2.7 |
240 |
0.16 |
D |
a. Which peak belongs to which compound in the final product? How can he confirm these assignments?
b. Determine the percentage composition in terms of isoborneol and borneol in the final product that all three compounds have the same response factor.
c. How would the spectrum change if the run would be started at T(initial)=80 oC and ends at T(final)=160 oC?
d. A student inject 2 mL instead of 1 mL of his sample? What would he observe?
e. Why is it important that the temperature of the detector is higher than the highest temperature used during the run?
2. A separatory funnel is used this week during the work-up. Answer the following questions.
a. Which tests should be performed prior to using the separatory funnel?
b. Why does the separatory funnel has a pear shape?
c. Why is it necessary to remove the plug on the top when draining the funnel?
d. Why is it important to vent the funnel frequently during extractions?
3. Referring to the phase transfer reaction carried out in the lab, answer the following questions.
a. What is the function of (NBu4)(HSO4) in this reaction?
b. What is the function of NaOCl in the reaction?
c. Why is disodium hydrogenphosphate added to the mixture?
d. Why is it important that the reaction mixture is stirred vigorously?
e. How could the student assess the completeness of the reaction prior the workup?
f. Why does benzil elute before benzoin during the column chromatography step?
PART II. (Reduction of Camphor, In-lab assignment)
This assignment is to be completed in the UCLA Science Learning Center computing labs during the lab period (or afterwards if you don't complete it in the allotted time). The assignment is due during meeting 5 and is worth 10 points.
1. Optimize the above structural geometry of borneol, camphor and isoborneol. (see below instructions). Compare the heat of formation of borneol and isoborneol. Which one is thermodynamically more stable? How can you rationalize the differences?
2. Determine the dipol moment for borneol, camphor and isoborneol. (see below instructions). In the GC spectrum, camphor has the shortest retention time, then isoborneol and borneol last. Can you rationalize the GC results based on the calculated dipole moments of these compounds? (Note: The GC column is relatively non-polar.) Do not apply any constraints in this part!
Instructions:
- Build, minimize and save the structure. (don't forget to Minimize the structure after building it!!)
 this is the minimize button. Always minimize your structure before leaving the building mode.
this is the minimize button. Always minimize your structure before leaving the building mode.
- click on
 to enter View Mode
to enter View Mode
- Select Calculations from the Setup menu.
The following window should appear. Select the options shown.
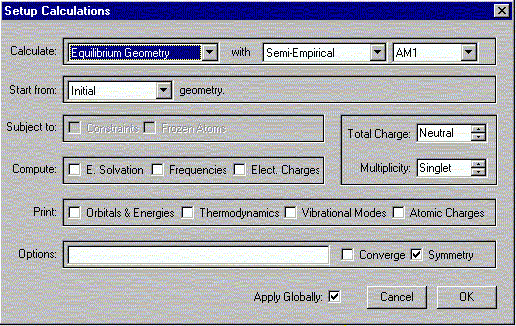
Verify the Charge is Neutral and Multiplicity is Singlet. Click OK.
- Select Submit from Setup menu.

- When the calculation is completed you will be notified.
- Select Output from the Display menu.
Record the heat of formation value given
See these Helpful Hints for manipulating structures!
PART III. (Aldol Condensation, In-lab assignment)
|
1. Calculate the dipole moment for dihedral angles:
0 through 180 degrees in 15 degree increments.
Plot the dipole moment of benzil vs. the dihedral angle.
|
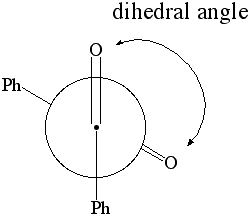
Newman Projection of Benzil
|
Instructions:
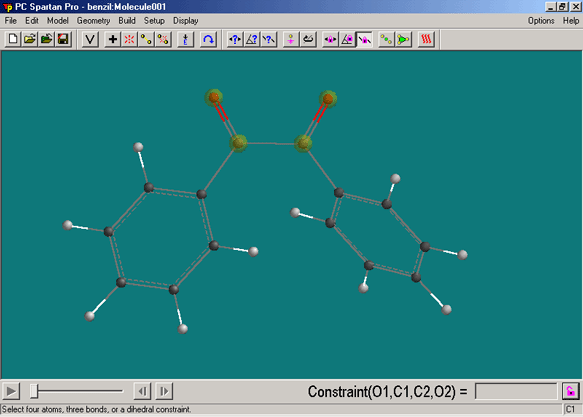
- click on Constrain Dihedral angle tool,

- select the atoms shown below in the specific sequence O..C..C..O. This will define two planes O1C1C2 and C1C2O2, which are going to be rotated towards each other in this part.
- Click on the lock tool,
 , in the bottom right corner so that it looks like this,
, in the bottom right corner so that it looks like this, 
- Set the dihedral angle to 0 and hit the ENTER key.
- click on the minimize tool,
 , and wait for the operation to complete.
, and wait for the operation to complete.
- click on the minimize tool,
 , a second time and wait for the operation to complete.
, a second time and wait for the operation to complete.
- Select Calculations from the Setup menu. The following window should appear. Select the options shown.

- Make sure the
- Click OK to close the window.
- Select Submit from Setup menu. When the calculation is completed you will be notified.
- Under the Display menu, select Properties.

- A window should appear (the values in this example should differ from yours). If there are no values in the window then you need to double click on the molecule.

- Record the dipole moment and energy given. (In this example the dipole = 3.9 debye).
- Calculate the dipole moment for dihedral angles: 0 through 180 degrees in 15 degree increments. (Hint: Just click on the pink bond and change the value in the box.). Do not define a new constraint at this point. This will confuse the program completely!
- For the higher angles (>120 degrees), you might have to uncheck the symmetry box in the setup calculation menu. However, sometimes it works better if the approach is for the higher angles is from 180 degrees going down.
- Using EXCEL, plot the dipole moment and energy of benzil vs. the dihedral angle (use MS Excel, XY Scatter plot). Rationalize the observed trends.
 this is the minimize button. Always minimize your structure before leaving the building mode.
this is the minimize button. Always minimize your structure before leaving the building mode. to enter View Mode
to enter View Mode






