updated last
Tuesday, October 06, 2015
Announcements (please read them carefully since you are going to held responsible)
1. Safety
a. Make sure to be careful when you handle glassware. This applies to its use during a reaction as well as the cleaning part. Broken glassware can cause very deep cuts, which have often to be stitched up (we had several cases of this over the years already). If chemicals get into the open wounds, the wounds have to be specially treated and the healing process usually takes longer than normal as well.
b. It has come to the attention of the instructor that students have been dumping acetone down the sink. This is entirely unacceptable. If a student will be caught doing this, s/he will receive an automatic zero for the entire lab meeting. Repeat offenders will be dismissed from the course on the account of being a safety risk. CAL OSHA will spell out very hefty fines for such violations because this is prohibited by California law!
c. The students are not allowed to use any reader in the lab. All information that is needed to carry out the experiment has to be included into the pre-lab. A student that uses the readers in the lab (for whatever reason) is deemed to be unfit for the lab and will receive a zero in-lab evaluation score for this lab meeting. The student will not be allowed to perform an experiment without a prelab!
d. Make sure to inform yourself about the hazards of the chemicals that you are using in the lab by reviewing the MSDS sheets for the chemicals used in the lab. It is your responsibilty to be informed about that for your own protection. Please keep in mind that you can also be tested about this knowledge by the TA, the instructor and any safety inspector i.e., departmental safety officer, EHS officer, CAL OSHA officer, reps from the City of Los Angeles, etc.
e. If you have to attend a different lab section, you will have to consult with the instructor. In the past, students attended a different lab section without permission, which poses a problem to everybody in the lab due to limited resources and safety issues (overcrowding of the lab, etc.). Failure to get permission from the instructor in the future will result in a 10-point penalty and automatic dismissal from the section.
f. The vacuum trap and the filter flask have to be clamped during the vacuum filtration.
2. Administrative Issues
a. It is important that the students leave the lab by 5:00 pm.
b. Each student has to submit the answers to his quizzes himself/herself. It is not acceptable that a fellow student does it under their name. This is considered cheating and will dealt with appropriately.
_______________________________________________________________________________________________________________________
Week 4 Problem Set - 30 BL (Turn in your computer assignment during meeting 5)
- Include Part I into your prelab.
- DO PARTS II and III DURING YOUR ASSIGNED LAB PERIOD WHILE THE REACTION IS STIRRING.
- ANSWERS TO ALL OF THESE QUESTIONS SHOULD BE IN YOUR LAB NOTEBOOK.
- This assignment is worth 10 points and should take about 45 minutes to complete. If you do not complete it during tihs time, you will have to come back later. This part is due at the beginning of meeting 5.
Part I: Phase Transfer Oxidation (due in prelab)
1. Please watch the following video (MP4-format) and read the appropriate chapter in the "Survival Kit Reader". Then take the quiz below.
Video: Extraction
Online Quiz: http://bacher.chem.ucla.edu/TakeQuiz/?id=c51ce410c124a10e0db5e4b97fc2af39
In order to take the quiz, you have to go through a UCLA ICP address. This means that you either have to use your Bruin-Online account or go through the VPN (Vitual Private Network, software can be found here: http://www.bol.ucla.edu/services/vpn/) to have this UCLA ICP address. If the video does not start automatically, you will have to download it first and then start it directly from Real Player or a similar program, etc.
To log in, use your last name and your student ID. If you are experiencing problems, contact the instructor via email and include your full name (indicated which one is your last name), your student ID, section and TA. (Hint: Think very careful about each response since many of the questions have more than one answer to them! Many students come up with the most obvious one and miss some of the details which leads to a zero score for the question!) Even though you can take the quiz until one hour prior to meeting 4 of your section, you should not delay taking it since there might be some problems with the server or the login. Also, there seem to be problems with MAC systems, Safari and Google Chrome Browser. The best is using IE 8.0 or Firefox. After you submit the answers, your score has to appear on your screen. If this does not happen, you will have to retake the quiz. (There will not be any possibility to retake the quiz weeks later since you are supposed to show preparedness at the point in time when you enter the lab!). The quiz is worth 10 points.
2. Referring to the phase transfer reaction carried out in the lab, answer the following questions.
a.
What is used as catalyst in the reaction? Show the key intermediate that involves this compound.
b. What is used as solvent in the reaction? Rationalize the
choice.
c. Why is it important to mix the reaction mixture properly?
d. Why is it important that the organic layer dried thoroughly
before performing the column chromatography step?
e. Sketch the TLC plate (alumina, ethyl acetate) for an
incomplete reaction (benzoin in lane 1, benzil in lane 2 and the
reaction mixture in lane 3).
f. Ethanol (95 %) is used as the solvent for the
recrystallization. Rationalize this choice.
g. After transferring the crystals to the Hirsch funnel, they are
rinsed. Which solvent is used here? How much of it?
h. The student submits a HPLC sample of his final product. Which solvent is used here? What should the concentration of the sample be?
How much of the solution is submitted?
i. Why is it important to observe "Rule 21"?
PART II. (Molecular Modeling II)
This assignment is to be completed in the UCLA Science Learning Center computing labs during the lab period (or afterwards if you do not complete it in the allotted time). The assignment is due during meeting 5 and is worth 15 points.
Instructions:
- Build, minimize and save the structure. (don't forget to Minimize the structure after building it!!)
 this is the minimize button. Always minimize your structure before leaving the building mode.
this is the minimize button. Always minimize your structure before leaving the building mode.
- click on
 to enter View Mode
to enter View Mode
- Select Calculations from the Setup menu.
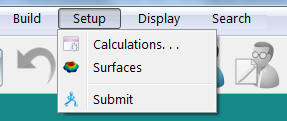
The following window should appear. Select the options shown.
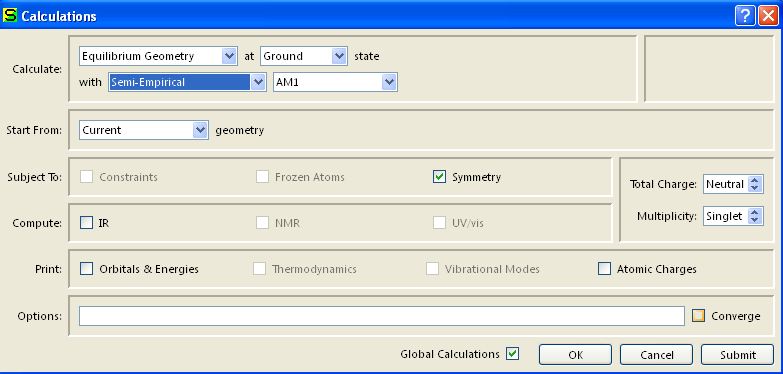
Verify the Charge is Neutral and Multiplicity is Singlet. Click OK.
- Select Submit from Setup menu.

- When the calculation is completed you will be notified.
- Select Output from the Display menu.
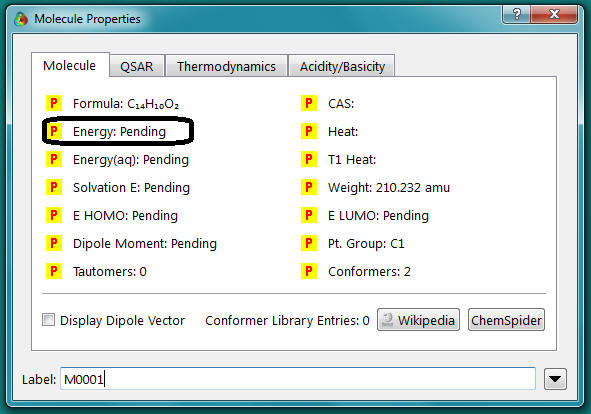
Record the heat of formation value given
See these Helpful Hints for manipulating structures!
PART IIa. (Aldol Condensation, In-lab assignment)
|
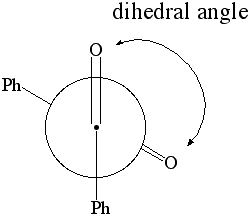
1. Calculate the dipole moment and the energy for dihedral angles:
0,
20,
40, 60, 90, 100, 105, 110, 120, 140, 160, 180
|
Newman Projection of Benzil
|
Instructions:
- Click on Constrain Dihedral angle
in the Geometry menu.
- select the atoms shown below in the specific sequence O..C..C..O. This will define two planes O1C1C2 and C1C2O2,
which are going to be rotated towards each other in this part
(Note that C1 and C2 are the two carbonyl carbon atoms)
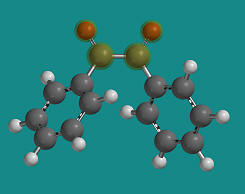
- Click on the lock tool,
 , in the bottom right corner so that it looks like this,
, in the bottom right corner so that it looks like this,

- Set the dihedral angle to 0 and hit the ENTER key.
- click on the minimize tool,
 , and wait for the operation to complete.
, and wait for the operation to complete.
- click on the minimize tool,
 , a second time and wait for the operation to complete.
, a second time and wait for the operation to complete.
- Select Calculations from the Setup menu. The following window should appear. Select the options shown.
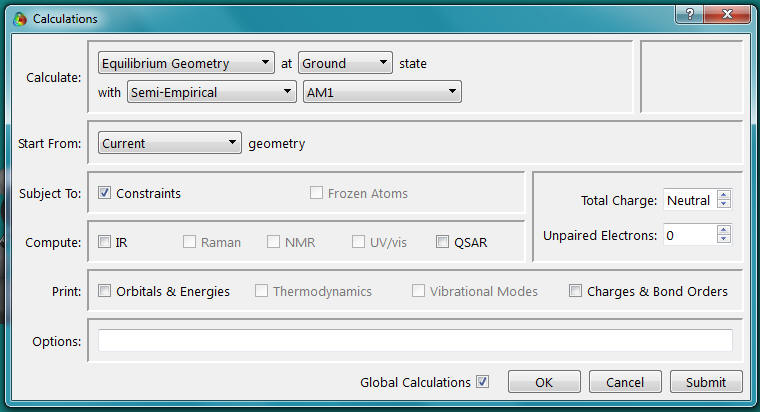
- Make sure the
 are checked!
are checked!
- Click OK to close the window.
- Select Submit from Setup menu. When the calculation is completed you will be notified.
Under the
Display menu, select Properties.
- A window should appear (the values in this example should differ from yours). If there are no values in the window then you need to double click on the molecule.
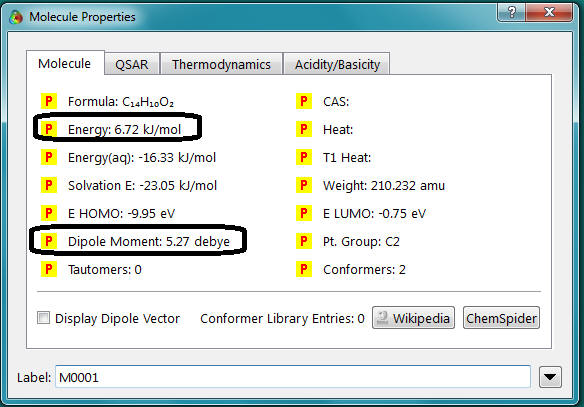
- Record the dipole moment and energy given. (In this example the dipole =
5.27 debye and the energy is 6.72 kJ/mol, the Energy(aq) is not important to us!) Hint: Just click on the pink bond and change the value in the box. Do not define a new constraint at this point. This will confuse the program completely!
- For the higher angles (>120o), you might have to uncheck the symmetry box in the setup calculation menu. However, sometimes it works better if the approach is for the higher angles is from 180o going down. In some cases, the symmetry feature has to be removed and put back in afterwards.
- Using EXCEL, plot the dipole moment and energy of benzil vs. the dihedral angle (use MS Excel, XY Scatter plot). Rationalize the observed trends.
PART IIb: (Infrared simulation, In-lab assignment)
Using the Hartree-Fock (3-21G) method of Spartan 2014, determine the carbonyl bond length in the following compounds (Do not apply any constraints in this part!):
| Compound |
ν[cm-1] |
| benzoyl chloride |
1774 |
| benzaldehyde. |
1703 |
| N,N-dimethylbenzamide |
1646 |
1. For the compound given in the table, plot the given carbonyl stretching frequency vs. the calculated C=O bond distance (Hint: Click on the C=O bond to obtain the bond length). Report the R-value and the equation of trend line. Rationalize the observed trend.
2. Based on the obtained graph in 1.,
predict the carbonyl stretching frequency of Propiophenone (C6H5COCH2CH3),
p-toluic acid (CH3C6H5COOH) and benzoyl
fluoride (C6H5COF). Comment on the results.
PART IIc: (UV-Vis simulation, In-lab assignment)
1. Data acquisition
a. Draw trans 1,2-dimethylethene (n=1, trans-butene). Minimize its energy. The
program should recognize the compound. Click on the little arrow
next to the name. A box will pop up with several calculations.
Check the box as shown below (B3LYP-6-31G*) and then
click Replace.
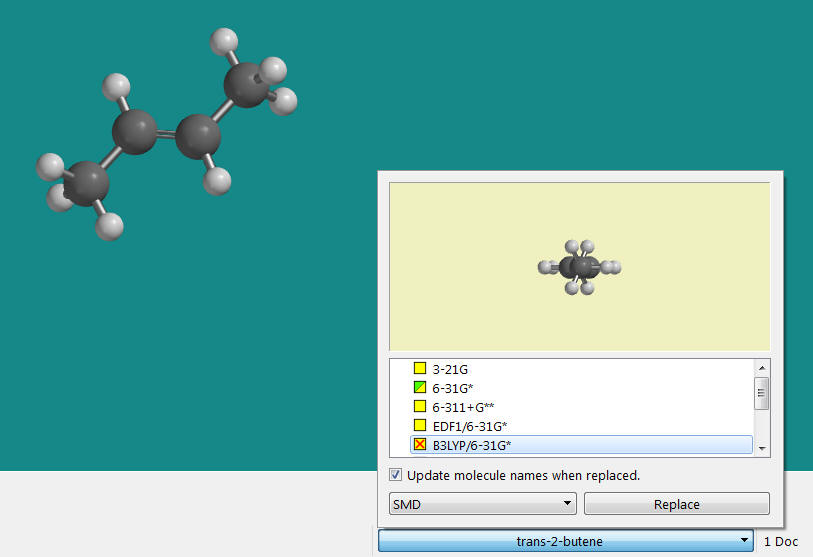
Determine the energies for the HOMO and the LUMO energies for
the molecule by using the Display-Properties feature.
b. Repeat the same for 2,4-hexadiene, 2,4,6-octatriene and
2,4,6,8-decatetraene. Make sure that double bonds are in trans configuration.
2. Data analysis
a. Based on the data obtained in 1., graph the HOMO and LUMO
energies in an energy diagram. Determine the energy for the
HOMO-LUMO gap. Rationalize the observed trends.
b. Determine the wavelength for the π-π* transition of the
compounds. Compare them with the literature provided in the SKR
(page 306).
 this is the minimize button. Always minimize your structure before leaving the building mode.
this is the minimize button. Always minimize your structure before leaving the building mode. to enter View Mode
to enter View Mode








