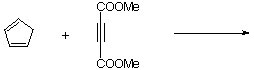
Week 4 Problem Set - 30BL
1. Referring to gas chromatography answer the following questions. Explain briefly.
a) define "retention time"
b) how would the retention time of borneol be affected if a more polar column was used?
c) how would the retention time of borneol be affected if the rate of flow of the carrier gas was decreased?
d) how would the retention time of borneol be affected if the column temperature was increased?
e) how would the quality of the separation be affected if the column temperature was increased?
f) how would the quality of the separation be affected if the flow rate of carrier gas was increased?
2. Assuming that a polar column is used explain the following sequence of retention times
cyclohexanone > cyclohexane > pentane
3. Ether or methylene chloride are commonly used solvents in GC-MS analysis. Explain.
4. If the column temperature or carrier gas flow rate is too high a mixture consisting of isoborneol and borneol will not be separated in the gas chromatograph. Explain.
6. Ligroin is a solvent used in this week's experiment (i.e., Diels-Alder rxn). What is ligroin and why is it used?
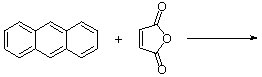
7. Predict the major expected product (with correct stereochemistry!) for the following Diels-Alder cycloadditions:
| a. | 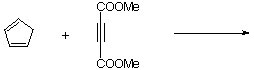 |
| b. |  |
| c. |
d. Suggest a synthetic route to obtain Aldrin via Diels-Alder reaction.
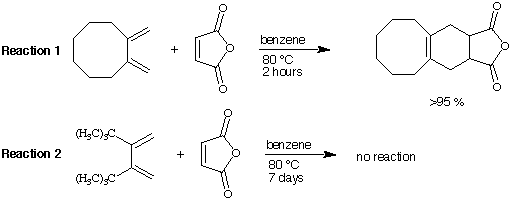
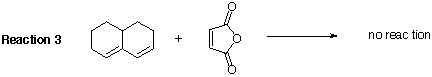
8. Cycloaddition of maleic anhydride to 1,2-dimethylenecyclooctane (Reaction 1) yields the Diels-Alder cycloadduct in less than 2 hours when refluxed in benzene. Cycloaddition of maleic anhydride to 2,3-di-tert-butyl-1,3-butadiaene (see Reaction 2) does NOT yield a cycloadduct even after reflux for 7 days in benzene. Reaction 3 and Reaction 4 do also not lead to the desired Diels-Alder product. EXPLAIN THE DIFFERENCE IN REACTIVITY.
![]()
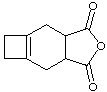
9. Supply the structure of the diene and dienophile needed to make the below compounds (don't answer this one for prelab assignment).
| a. | b. |
 |
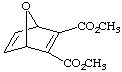 |