Meeting 3 Problem Set - 30BL
Announcements
1. NO ONLINE QUIZ FOR THIS MEETING!
2. The IR assignment for this quarter is due on January 22, 2010 at 5:00 pm in YH 3077E. No late assignments will be accepted. Assignments cannot be submitted via email, fax, etc.
Homework questions for meeting 3 (all part are part of the prelab!)
1. Using PC Spartan 2002 at the Science Learning Center (4th Floor Young Hall) do the following:
a. Draw camphor if you have not done it yet (Make sure that all hydrogen atoms are still attached!). Minimize the structures by clicking the minimize icon, ![]() , then save.
, then save.
b. Go to the view mode by clicking the view icon, ![]() . Once in the view mode, click the Model menu and select Tube.
. Once in the view mode, click the Model menu and select Tube.
c. Select Calculations from the Setup menu.
The following window should appear. Select the options shown.
Verify the Charge is Neutral and Multiplicity is Singlet. Click OK.
- Select Submit from Setup menu.
- Under the Setup menu, select Surfaces. A window should appear. In this window click Add.
A second window should appear. In this window, select the following
Then click OK to exit dialog. Close the previous window.
Select Submit from Setup menu. When the calculation is completed you will be notified.
- Under the Display menu, select Surfaces.
A window should appear. In this window check the yellow box.
Next, select the molecule by clicking on it. In the bottom right corner of the window, change the selection from Solid to Transparent.
d) Do the same (steps a-c) for 2-norbornanone.
2. Referring to the below reaction,
answer the following questions:
a. Which isomer is expected to be the major product for this reaction? Why? (Include the LUMO diagram generated in question 1 to answer this question. Make sure to show the proper orientation! Hint: What are you trying to show here?)
b. What would change if NaBD4 and CD3OD/D2O were used in this reaction? Show the structure of the product.
c. Why is the LUMO orbital used to rationalize the stereochemistry in this reaction?
3. Referring to the below reaction,
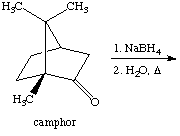
answer the following questions:
a. Draw the products formed in this reaction
b. Which product is the major and which is the minor? Explain using the LUMO generated above (Include the LUMO diagrams generated in question 1 to answer this question. Make sure to show the proper orientation!). What are the differences compared to 2-norboranone?
c. How would the outcome of the reaction change if LiBH(n-Bu)3 was used instead of NaBH4? Explain.
4. Experimental
a. Why is methanol is used as solvent in the reaction? Why is this not an optimal approach?
b.Why is it important the solvent is present during the reaction?
c. After the reaction is completed, the reaction mixture is chilled and water is added. Which purpose does this step serve?
d. The amount of drying agent used here has to be minimized. Explain why. How does the student know that he added enough?
e. Why is the solvent removed using a warm water bath in the hood as compared to just boiling it off on the hotplate?
f. A student observes a melting point of 202-208 oC for his final product in this reaction. Which conclusion(s) can he draw?
g. Which information does the student obtain from the GC spectrum?
h. What are the changes in the FTIR spectrum going from camphor to isoborneol/borneol? Provide the functional groups and their corresponding wavelengths.
i. In which way would the FTIR spectra of isoborneol differ, if they were acquired in the solid or the gas phase? Explain.