Meeting 3 Problem Set - 30BL
Announcements
1. Safety and other in-lab issues
a. The student has to come properly dressed to the lab. Long pants and closed-toed shoes are mandatory. A lab coat of proper size has to be worn all at times in the lab. The lab coat has to be closed as well. In addition, goggles have to be carired at all times. Failure to follow these rules will lead to a dismissal from the course.
b. Upscaling of reactions is strictly prohibited. Anybody doing so will be reported to the Dean of Students for cheating. There are reasons why the experiments are carried out in the scale given in your course reader (safety, econonics, etc).
c. All wash bottles have to be properly labeled with their content i.e water, acetone, etc.
2. NO ONLINE QUIZ FOR THIS MEETING!
3. The IR assignment for this quarter is due by August 20, 2010 at 5:00 pm in YH 3077E. No late assignments will be accepted. Assignments cannot be submitted via email, fax, etc either.
----------------------------------------------------------------------------------------------------------------------------------------------------------------
Homework questions for meeting 3 (all part are part of the prelab!)
1. Using PC Spartan 2008 at the Science Learning Center (4th Floor Young Hall) do the following:
a. Draw camphor if you have not done it yet (Make sure that all hydrogen atoms are still attached!). Minimize the structures by clicking the minimize icon, ![]() , then save.
, then save.
b. Go to the view mode by clicking the view icon, ![]() . Once in the view mode, click the Model menu and select Tube.
. Once in the view mode, click the Model menu and select Tube.
c. Select Calculations from the Setup menu.
The following window should appear. Select the options shown.
Verify the Charge is Neutral and Multiplicity is Singlet. Click OK.
- Select Submit from Setup menu.
- Under the Setup menu, select Surfaces. A window should appear. In this window click Add.
A second window should appear. In this window, select the following
Then click OK to exit dialog. Close the previous window.
Select Submit from Setup menu. When the calculation is completed you will be notified.
- Under the Display menu, select Surfaces.
A window should appear. In this window check the yellow box.
Next, select the molecule by clicking on it. In the bottom right corner of the window, change the selection from Solid to Transparent.
d) Do the same (steps a-c) for 2-norbornanone.
2. Referring to the below reaction,
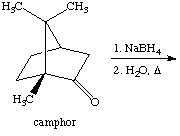
answer the following questions:
a. Draw the products formed in this reaction
b. Which product is the major and which is the minor? Explain using the LUMO generated above (Include the LUMO diagrams generated in question 1 to answer this question. Make sure to show the proper orientation! Hint: What are you trying to show here?).
c. How would the outcome of the reaction change if LiBH(n-Bu)3 was used instead of NaBH4? Explain.
d. What would change if NaBD4 and CH3OD/D2O were used in this reaction? Show the structure of the product.
3. Referring to the below reaction,
answer the following questions:
a. Which isomer is expected to be the major product for this reaction? Why? (Include the LUMO diagram generated in question 1 to answer this question. Make sure to show the proper orientation! Hint: What are you trying to show here?)
b. What would changed if there was a methyl group placed in 1-position?
4. Experimental
a. The procedure asks to use to methanol. Why is this solvent chosen? Which problem does this choice pose?
b.Why is it important that the solvent is present during the reaction?
c. After the reaction is completed, the reaction mixture is chilled and cold water is added. Which purpose does this step serve?
d. How does the student know that enough drying agent was added?
e. The solvent is removed using a rotary evaporator. Which advantages does this method of solvent removal provide?
f. How much sample is used for the determination of the melting point?
g. Why is it important to prepare a fairly dilute sample for the acquisition of the GC spectrum? Which solvent is used here?
h. Where can the GC spectrum for your sample be picked up?