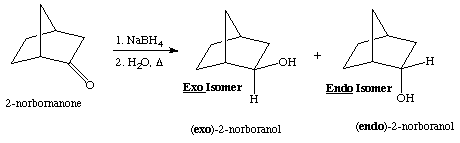 (endo/exo huh?)
(endo/exo huh?)Meeting 3 Problem Set - 30BL
1. Referring to the below reaction,
a) which isomer is expected to be the major product for this reaction? why? Draw a sketch of the LUMO diagram to answer this question.
b) what would change if LiAlD4 and H2O are used in this reaction?
2. Referring to the below reaction,
a) draw the products formed in this reaction
b) which product is the major and which is the minor? Explain using the LUMO diagram.
c) what would change if NaBH4 and D2O are used in this reaction?
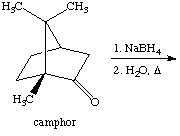
3. Referring to the reduction of camphor answer the following questions.
a. Methanol used as a solvent for the reaction. Explain briefly why.
b. How many moles of of NaBH4 are required (in theory) to reduce 4.5 mmol of camphor? Show pertinent chemical equation(s).
c. Why is the diethyl ether boiled off in a water bath, and not straight on the hotplate?
d. What does the term 'decant' refer to?
e. Why should the sodium borohydride bottle be kept closed if not needed?
f. Outline a brief procedure how the melting point is acquired for the final product? Which information can be gathered from the data?
4. The IR spectrum is acquired for the final product of the reaction. Outline a brief procedure how the IR sample for a solid is prepared.