Problem set #2 (Meeting 2)
Announcements
1. You are not allowed to use any readers in the lab starting meeting 2. All information that is needed to carry out the lab should be in your pre-lab report. No exceptions!
2. You will not be permitted to carry out any experiments in the future without proper attire! This means if you do not show up in proper clothing or/and do not wear goggles and a lab coat in the lab, you will be excused from the lab with a zero score (lab performance and quiz!) for this lab the first time (this means that you still will have to make up the lab later on withgout getting any credit for it!). The second time, the consequences will be more severe i.e. dismissal from the course due to safety issues.
ATTN: answers to the below questions are due at the start of meeting 2; these answers should be part of your pre-lab write-up. Show pertinent equations where appropriate.
1. Referring to the benzoin condensation, answer the following questions.
a. After the thiamine hydrochloride is dissolved in water, 2N sodium hydroxide solution is added. Provide a chemical equation for this reaction. What should the student observe?
b. Why is imperative that 95% ethanol is added to the mixture obtained in a. before adding the benzaldehyde?
c. The reaction mixture is subsequently sealed with parafilm, labeled and stored in the drawer. Rationalize these steps.
d. The student returns to the lab two days later to check on his reaction. Unfortunately, the solution did not yield any precipitate. :-( What should he do at this point?
e. What is an ice-bath?
f. A student starts with 0.20 g of thiamine hydrochloride and 3.5 mL of 0.20 N sodium hydroxide solution. Afterwards, he adds 2.0 mL of benzaldehyde to the solution. After the work-up, he isolates 1.20 g of white crystals. Determine the yield for the reaction. Show your calculations.
g. The student comes to office hours and tells the instructor that the product of the reaction has a melting point of 127 oC. Which conclusion can the instructor make about the isolated compound and the student's knowledge?
2. Vacuum filtration
Please watch the following video (MP4-format) and read the appropriate chapter in the "Survival Kit Reader". Then take the quiz below.
Online Quiz (will most likely not be active prior January 10, 2009 due to enrollment issues)
http://bacher.chem.ucla.edu/TakeQuiz/?id=c9f0f895fb98ab9159f51fd0297e236d
In order to take the quiz, you have to go through a UCLA ICP address. This means that you either have to use your Bruin-Online account or go through the VPN (Vitual Private Network, software can be found here: http://www.bol.ucla.edu/services/vpn/) to have this UCLA ICP address.
To log in, use your last name and your student ID. If you are experiencing problems, contact the instructor via email and include your full name (indicated which one is your last name), your student ID, section and TA. (Hint: Think very careful about each response since many of the questions have more than one answer to them! Many students come up with the most obvious one and miss some of the details which leads to a zero score for the question!) Even though you can take the quiz until one hour prior to meeting 2 of your section, you should not delay taking it since there might be some problems with the server or the login. Also, there seem to be problems with MAC systems and Safari Browser. The best is using IE 7.0 or Firefox. After you submit the answers, your score has to appear on your screen. If this does not happen, you will have to retake the quiz. (There will not be any possibility to retake the quiz weeks later since you are supposed to show preparedness at the point in time when you enter the lab!). The quiz is worth 10 points.
3. Referring to the elimination reaction, answer the following questions.
a. What is the function of the conc. phosphoric acid in the reaction? What is conc. phosphoric acid anyway?
b. Why are conc. phosphoric acid and conc. sulfuric acid fairly viscous at room temperature?
c. Which compound(s) is(are) collected during the first distillation if the distillation was carried out properly? Where are they collected?
d. Why is saturated sodium chloride solution used for in this experiment?
e. Why is it important that to remove the sodium sulfate after the drying step is completed?
f. Why is it unwise to distill the crude product entirely?
g. One of qualitative tests that is performed to verify the presence of an C-C double bond is the reaction with potassium permanganate. Show the pertinent chemical equation (including proper stereochemistry). What does the student observe in this reaction when using cyclohexene and cyclohexanol, respectively?
4. a. Using PC Spartan 2002 (which is only available in the SLC), perform the following calculations for the alkenes: terpinolene, limonene, a-terpinene, isoterpinolene. Make sure that all hydrogen atoms are present and there are also no extra ones either.
b. Select Calculations from the Setup menu.

The following window should appear. Select the options shown.
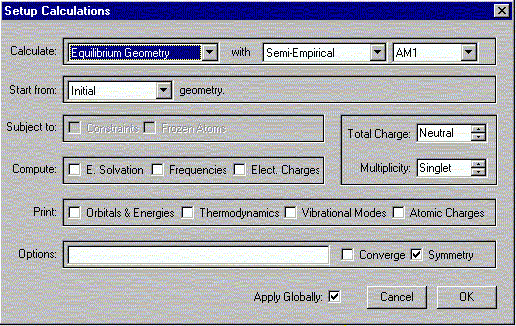
Verify that the Charge is Neutral and Multiplicity is Singlet. Click OK.
Select Submit from Setup menu.

Then click OK to exit dialog. Close the previous window.
Select Submit from Setup menu. When the calculation is completed you will be notified. It should not take more than a minute for the calculation to complete. Make sure that you have only one window open at this point. The messages are usually displayed in the first window, and often not in the window you are working in at this point.
Under the Display menu, select Properties. Record the value for the Energy.
Data Analysis:
1. Based in the calculated trends in the heat of formation (=energy), which alkene appears to be the most stable based on the calculation? How does this data match with the heats of formation given on page 21 (which are the experimental data)? Can you rationalize the differences?
2. Can you explain the product distribution observed for the elimination of water from a-terpinol (see reader page 20)?
3. Which conclusions can be drawn from this simulation?
Problems with calculations: Check here