Problem set #2 (Meeting 2)
ATTN: answers to the below questions are due at the start of meeting 2; these answers should be part of your pre-lab write-up. Show pertinent equations where appropriate.
1. The formation of alkenes from primary alcohols requires more rigorous conditions than the elimination from secondary alcohols. Rationalize this observation.
2. Why is the mixture heated during the reacion?
3. A saturated sodium chloride solution is added during the work-up. Explain briefly why.
4. Infrared spectroscopy
a. What are the most important changes in the IR spectrum going from cyclohexanol to cyclohexene (functional groups and wavenumbers)?
b. How are you going to acquire the IR spectrum for the final product in the lab? Outline a brief procedure.
5. Referring to the benzoin condensation, answer the following questions:
a. Why is it important to have fresh benzaldehyde for this reaction? How do you know that the aldehyde is 'old'?
b. Why is it important that the reaction mixture is homogeneous?
6. Vacuum fitration
a. Draw a sketch for the setup and label all parts needed.
b. Why is it important to use a filter paper with the correct size?
c. Why should the filter flask be clamped during the filtration?
d. Explain briefly the differences between a vacuum filtration and a gravity filtration.
7. Using PC Spartan 2002 at the Science Learning Center (4th Floor Young Hall) do the following:
a. Do the following calculations for the alkenes: terpinolene, limonene, a-terpinene, isoterpinolene. Make sure that all hydrogen atoms are present.
b. Select Calculations from the Setup menu.

The following window should appear. Select the options shown.
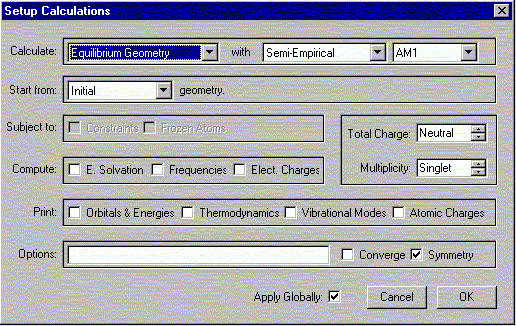
Verify that the Charge is Neutral and Multiplicity is Singlet. Click OK.
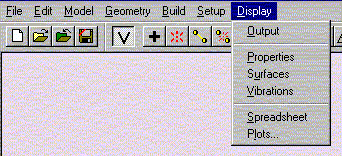
Select Submit from Setup menu.

Then click OK to exit dialog. Close the previous window.
Select Submit from Setup menu. When the calculation is completed you will be notified. It should not take more than 2-3 minutes for the calculation to complete under normal circumstances. Make sure that you have only one window open at this point. The messages are usually displayed in the first window, and often not in the window you are working in at this point.
Under the Display menu, select Properties. Record the value for the Energy.
1. Based in the observed trends in trends in the heat of formation (=energy), which alkene is the most stable and which one is the least stable one? Can you rationalize these observations?
2. Can you explain the product distribution observed for the elimination of water from a-terpinol (see page 22)?
Problems with calculations: Check here