Problem set #2 (Meeting 2)
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. What drives the reaction towards the elimination product?
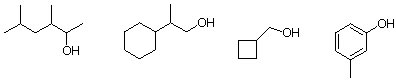
2. What is the expected alkene product formed from the dehydration of the following alcohols? Show major products only. help

3. In the lab, you will conduct two qualitative tests to determine the presence of a double bond in the final product. Show the equations including the proper stereochemistry of the products.
4. How can IR spectroscopy help you to evaluate the success of the reaction?
5. Why do you need more rigorous conditions to eliminate water from n-propanol than from neo-pentanol?
6. What is the function of the sodium hydroxide solution in the benzoin experiment? What do you observe upon addition?
7. Why is it important to use fresh benzaldehyde? How can you purify benzaldehyde? (Hint: What happens if you store aldehydes for longer time?)
8. .Using PC Spartan at the Science Learning Center (4 Floor Young Hall) do the following:
a. Use the alcohols and their protonated forms that you drew last week for this exercise.
b. Select Calculations from the Setup menu.
The following window should appear. Select the options shown.
Verify the Charge is Neutral (for -OH) or Charge is Cation (for -OH2) and Multiplicity is Singlet. Click OK.
- Select Submit from Setup menu.
- Then click OK to exit dialog. Close the previous window.
- Select Submit from Setup menu. When the calculation is completed you will be notified.
- Using the distance tool, measure the distance C-O and record it.
- Under the Display menu, select Properties. Click on the oxygen atom.A new window should appear (atom properties). Record the value for the Natural Charge. Do the same for the C-atom the has the oxygen attached.
Based on the obtained data, answer the following questions:
a. How does an added proton influence the C-O-bond distance in the different molecules? Rationalize the changes.
b. How does it influence the partial charges on the carbon and oxygen?