Problem set #2 (Meeting 2)
Announcements (Please sure to read them carefully since you will be held to them)
1. You will not be allowed to use any readers in the lab starting meeting 2. All information that is needed to carry out the lab should be in your pre-lab report. No exceptions! You will not be permitted to perform any experiment without a proper prelab.
2. You will not be permitted to carry out any experiments without proper attire! If you do not show up in proper clothing or/and do not wear goggles, a proper length flame-resistant lab coat and nitrile gloves in the lab (read UCLA PPE policy), you will be excused from the lab with a zero score (lab performance and quiz!) for this lab the first time (you still will have to make up the lab later on without getting any credit for it!). The second time, the consequences will be more severe i.e., dismissal from the course for not following the safety protocol. CAL OSHA and similar offices have been inspecting the department a lot recently (unannounced) and have passed out very heavy fines (i.e., not wearing a proper lab coat, goggles or nitrile gloves costs more than $1000 per incident which will come out of your bar account if you are caught by them!).
3. The infrared assignment for this quarter is posted (see link on front page). The assignment is due by April 17, 2015 at 4:30 pm in the instructor's office in YH 3077E. Late assignments will not receive any credit. Assignments submitted via email will not be accepted either. If you are not on campus on Friday, you will have to submit the assignment early. Plan your workload accordingly. (The instructor will not allow you to drop the course if you failed to turn in the assignment.) You cannot retrieve the assignments after you submitted it.
4. Make sure that you have access to the online quiz system as soon as possible. If not, inform the instructor asap. This system is largely independently operated from URSA, which means that even if you are enrolled in the course, you might not be in the database for the quiz system.
5. Submit two separate pre-labs during meeting 2: one for the benzoin experiment and one for the elimination experiment.
ATTN: The answers to the questions below are due at the start of meeting 2; these answers should be part of your pre-lab write-up. Show a balanced chemical equation and proper stereochemistry where appropriate.
1. Referring to the benzoin condensation, answer the following questions (include into the benzoin prelab).
a. A student uses ethanol for the initial part of the reaction. What would he observe?
b. Thiamine hydrochloride is reacted with sodium hydroxide. Provide a balanced equation for the reaction.
c. After benzaldehyde is added to the solution obtained in b., the flask has to be sealed. How is this accomplished and why is this important?
d. A student starts with 0.30 g of thiamine hydrochloride and 2.75 mL of 0.10 N sodium hydroxide solution. Afterwards, he adds 2.40 mL of benzaldehyde to the solution. After the work-up, he isolates 2.10 g of white crystals. Determine the percentage yield for the reaction. Show your calculations.
2. Vacuum filtration (sorry the link to the quiz will not be active before 4/2/2015 (around 2 pm) due to enrollment)
Please watch the following video (MP4-format) and read the appropriate chapter in the "Survival Kit Reader". Then take the quiz below.
Hint: If the video does not start, download the file to the desktop and open it directly from a video player (i.e., Real Player)
Online Quiz
http://bacher.chem.ucla.edu/TakeQuiz/?id=c9f0f895fb98ab9159f51fd0297e236d
In order to take the quiz, you have to go through a UCLA ICP address. This means that you either have to use your Bruin-Online account or go through the VPN (Virtual Private Network, software can be found here: http://www.bol.ucla.edu/services/vpn/) to have this UCLA ICP address.
To log in, use your last name and your student ID. If you are experiencing problems, contact the instructor via email and include your full name (indicated which one is your last name), your student ID, section and TA. (Hint: Think very careful about each response because many of the questions have more than one answer to them! Many students come up with the most obvious one and miss some of the details which leads to a zero score for the question because the system is not programmed to give partial credit!) Even though you can take the quiz until one hour prior to meeting 2 of your section, you should not delay taking it since there might be some problems with the server or the login. There seem to be problems with MAC systems, the Safari Browser and the Google Chrome Browser. The best is using IE 8.0 (or above) or Firefox. After you submit the answers, your score has to appear on your screen. If this does not happen, you will have to retake the quiz. (There will not be any possibility to retake the quiz weeks later since you are supposed to show preparedness at the point in time when you enter the lab!). The quiz is worth 10 points.
3. Referring to the elimination reaction, answer the following questions (include into the prelab for the elimination reaction).
a. Why is it important to use a catalyst in the reaction? Show the key intermediate.
b. Why can the approach that is used in the lab considered environmentally more benign than the use of concentrated sulfuric acid?
c. In which way does the student take advantage of the Le Châtelier principle? Why is this necessary?
d. Why is it important that the reaction mixture is heated for 15-20 minutes before the first distillation is started?
e. Nothing seems to distill over despite the hotplate being turned on and the thermometer of the hotplate providing a reading of 200 oC. How can the student troubleshoot the problem?
f. After the distillation was completed, the Hickman head is rinsed with saturated sodium chloride solution. The rinse is combined with the distillate. How is this mixture separated? Which layer contains the product?
g. How the infrared spectrum of the reactant and the product differ from each other? Provide key functional groups and wavenumbers.
h. Why is it important to observe Rule 8 in the lab?
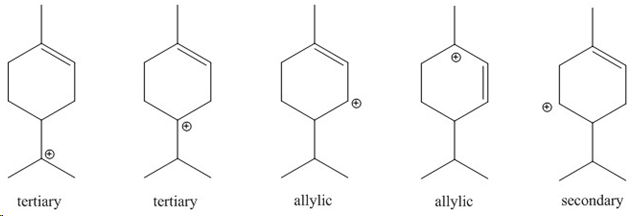
4. a. Using PC Spartan 2010 (which is only available in the SLC), perform the following calculations for the alkenes: terpinolene, limonene, a-terpinene, isoterpinolene and five cations (see below) that can potentially be formed during the elimination reaction from a-terpineol.

Make sure that all hydrogen atoms are present and there are also no extra hydrogen atoms either.
b. Select Calculations from the Setup menu.
The following window should appear. Select the options shown.
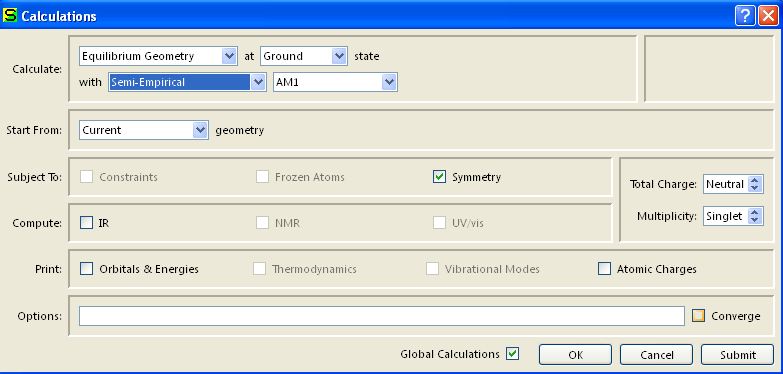
Calculate the energy of the Equilibrium Geometry at Ground State using Semi-Empirical, AM1 method. Verify that the Total Charge is Neutral (for the neutral molecules and Cation (for the cations) and Multiplicity is Singlet. Do not check the boxes for IR, Orbitals & Energies and Atomic Charges, because this will cause the calculation to take much more time! Select Submit from Setup menu. When the calculation is completed you will be notified by a pop-up window. It should not take more than a couple of seconds for the calculation to complete. Make sure that you have only one window open at this point. The messages are usually displayed in the first window, and often not in the window you are working in at this point. Under the Display menu, select Properties. Record the value for the Energy.
Data Analysis (include into the elimination prelab):
1. Outline a mechanism to show how the five cations above are formed.
2. Based in the calculated trends in the heat of formation (=energy), which alkene appears to be the most stable based on the calculation? How does this data match with the heats of formation given on page 21 (which are the experimental data)? Can you rationalize the differences?
3. Can you explain the product distribution observed for the elimination of water from a-terpineol (see reader page 21)?
4. Which conclusions can be drawn from this simulation? (Hint: Is the used computer model suitable to predict the outcome of the elimination experiment?)
Problems with calculations: Check here