Problem set #2 (Meeting 2)
ATTN: answers to the below questions are due at the start of meeting 2; these answers should be part of your pre-lab write-up. Show pertinent equations where appropriate.
1. Referring to the benzoin condensation, answer the following questions.
a. Why is it necessary to add sodium hydroxide solution in this reaction?
b. Why is it important to follow the sequence of additions in the experimental protocol?
c. How can "old" benzaldehyde be purified?
d. How many grams of benzoin are isolated in this reaction if the reaction typically runs in 75% yield? Show your calculations.
e. What are the most significant changes in the IR spectrum going from benzaldehyde to benzoin?
2. Vacuum fitration
a. Draw a sketch for the setup used for the filtration of the benzoin and label all parts needed.
b. Why is it important to use thick-walled tubing here?
c. Why is the filter flask clamped during the filtration?
3. Referring to the elimination reaction, answer the following questions.
a. Why is the mixture heated during the reaction?
b. Which layer is removed from the conical vial during the extraction/washing process? How is this accomplished?
c. Why is the crude product distilled again?
d. How are you going to acquire the IR spectrum for the final product in the lab? Outline a brief procedure.
e. Which qualitative tests are perfomed to establish the presence of the C=C bond in the product?
4. a. Using PC Spartan 2002, perform the following calculations for the alkenes: terpinolene, limonene, a-terpinene, isoterpinolene. Make sure that all hydrogen atoms are present.
b. Select Calculations from the Setup menu.

The following window should appear. Select the options shown.
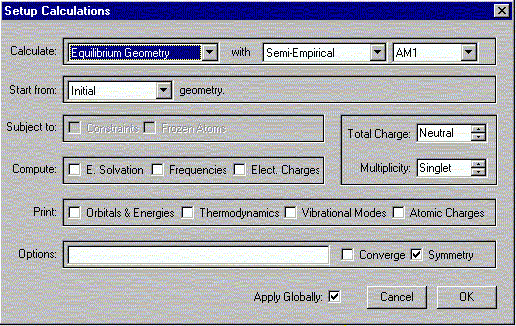
Verify that the Charge is Neutral and Multiplicity is Singlet. Click OK.
Select Submit from Setup menu.

Then click OK to exit dialog. Close the previous window.
Select Submit from Setup menu. When the calculation is completed you will be notified. It should not take more than one minute for the calculation to complete under normal circumstances. Make sure that you have only one window open at this point. The messages are usually displayed in the first window, and often not in the window you are working in at this point.
Under the Display menu, select Properties. Record the value for the Energy.
1. Based in the observed trends in trends in the heat of formation (=energy), which alkene is the most stable and which one is the least stable one? Can you rationalize these observations? Do the obtained data match with your expectation? If not, what is the conclusion?
2. Can you explain the product distribution observed for the elimination of water from a-terpinol (see reader page 20)?
Problems with calculations: Check here