Answer Key Meeting 6 (Diels-Alder-Reaction)
1. Online quiz (no key...consult the instructor for questions)
2. a. Benzyne is the dienophile and 1,2,3,4-tetraphenylcyclopentadienone is the diene in this Diels-Alder reaction.
b. Benzyne is obtained by the reaction of anthranilic acid (2-Aminobenzoic acid) and isopentyl nitrite. Aside of benzyne, water, isopentyl alcohol, carbon dioxide and nitrogen are formed in this reaction.
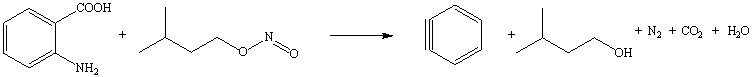
c. 1,2-Dimethoxyethane (H3COCH2CH2OCH3) is used as solvent because it is able to dissolve the polar anthranilic acid (log Kow=1.21) better than most other solvents i.e., diethyl ether. Its higher polarity (log Kow= -0.21) compared to diethyl ether (log Kow=0.89) leads to a higher boiling point, which also allows the experimenter to raise the temperature in the reaction mixture. This is favorable because this reaction is entropy driven (three moles of gases are produced!) and it provides sufficient energy to decompose the intermediate.
d. The addition of isopentyl nitrite leads to the formation of CO, CO2 and N2 as byproducts of the reaction. The formation of these gases causes the mixture to foam heavily. If the isopentyl nitrite was added too fast, the reaction mixture can be expelled from the vessel. Since the solvent used in the reaction is flammable, this can lead to a fire. A high concentration of benzyne also leads to an increased amount of biphenylene.
e. If the reaction proceeds according to plan, the reaction mixture will foam and the color changes from purple to yellow-orange during to the consumption of the limiting reagent, TPCP.
f. The reaction mixture is added to a mixture of water and methanol (5:2) to precipitate the non-polar products (TPN, TPCP, biphenylene, bicyclic ketone intermediate). Addition of the reaction mixture to the solvent mixture allows the mixture to dissolve/suspend better leading to a precipitate that is easier to filter. If the solvent mixture is added to the reaction mixture, the precipitate is often very gummy because it does not break up well trapping a lot of impurities.
g. Isopropanol is used as solvent in the recrystallization. The crude dissolves reasonable well at high temperature and poorly at low temperature. The impurities remain dissolved in this solvent. However, the dissolution process itself is very slow.