Week 5 Condensed Answer Key
1. a. The
use of 95 % ethanol is not advisable in this experiment. Absolute
ethanol is used as solvent in the experiment. The main difference between absolute ethanol and 95 % ethanol is that the latter one contains about 4.5 % of water.
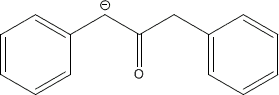
The presence of the water causes the equilibrium of the enolate formation
to be shifted further to the left which is very detrimental to the
reaction.
![]()
Ketone + OH- ←→ Enolate + H2O
This shift would reduce the concentration of the enolate in the reaction mixture, which reduces the rate of the reaction to a point at which very little product would be formed. At best, the student will observe a slight color change to dark-red or brown, but no precipitation.
b. The hydroxide ion is the catalyst in this reaction that converts some (about 1 % because the hydroxide ion is a fairly weak base) of the dibenzyl ketone into its enolate that subsequently acts as the nucleophile in the reaction.
c. The color of the reaction mixture changes from bright yellow to dark purple during the reaction (usually immediately after the base is added). After a couple of minutes of reflux, small, dark crystals appear on the upper part of the solution because the weakly polar compound starts to precipitate from the polar solvent. In many cases, the solution will start to bump as well if the spin vane is improperly placed and/or used in the vial.
d. Scraping the solid out of the vial is usually not very
efficient. Thus, the conical vial is inverted and ice-cold 95 % ethanol
is injected using the syringe to rinse the product out of the vial.
e. In the lab, a solvent mixture of 95 % ethanol and toluene
(1:1) is used as the solvent for recrystallization. TPCP dissolves well
at elevated temperatures but relatively poorly at low temperature in
this solvent mixture. Toluene as a single solvent is a poor choice as solvent for
recrystallization because
the compound is relatively low in polarity and dissolves well at low
temperature. Thus, it would be very difficult to recover the solid upon cooling ("like-dissolves-like").
As single solvent, ethanol is also a poor choice because the solubility
of the compound is too low at high temperatures.
f. Based on the polarity, TPCP should usually move the farthest and dibenzylketone the least. This applies particularly to low polarity solvent like hexane because they dissolve the low polarity compounds the best. The spots will be at the lower end of the TLC plate for heptane but move much more for diethyl ether. The separation will most likely be poor in both cases. The situation because more complicated for propanol because the lower polarity compounds display a low solubility in this solvent. TPCP will most likely be the lowest spot here despite the high eluting power of the solvent.
f. Rule 36 states
The solubility of a compound is usually the highest at the boiling point of the solvent. Since the amount of solvent used in the recrystallization should be minimize to optimize the amount of recovered product, the mixture should be heated to a boil.
g. The signal at m/z=356 is obtained after the loss of a CO molecule resulting in tetraphenylbutadiene (C4Ph4). This fragment decomposes to form diphenylacetylene (PhC≡CPh) with a mass of m/z=178.