 |
 |
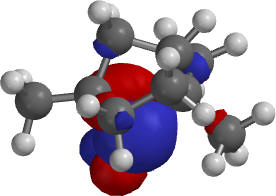
| D-Fenchone, LUMO endo approach (across the C2-bridge on the other side of the molecule) |
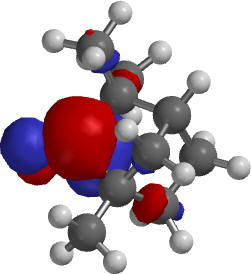
D-Fenchone, LUMO exo approach across the C1-bridge with the methyl groups |
Meeting 3 Problem set - CONDENSED KEY
The main point of generating the diagrams is to rotate them accordingly in order to be able to evaluate the steric effect of the different parts in the molecule.
1.The LUMO for D-fenchone is needed to rationalize the product distribution:
 |
 |
| D-Fenchone, LUMO endo approach (across the C2-bridge on the other side of the molecule) |
D-Fenchone, LUMO exo approach across the C1-bridge with the methyl groups |
The endo approach is more sterically encumbered. One has to compare the impact of different parts of the molecule on the approach of the nucleophile. Note that the lateral view is not helpful to assess the steric hindrance of the C2-bridge on the backside.
2.a.
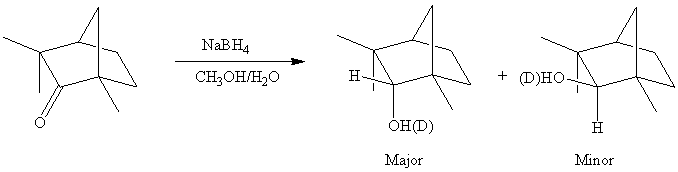
The reaction leads to the formation of four compounds because the products can contain either an "OH"- or an "OD"-group.
b. The endo product is the major product in the reduction of D-fenchone, which is the product formed by the exo approach (approach from above) of the nucleophile. The steric hindrance on the lower part (caused by the other long bridge above) of the molecule is larger. The approach of the nucleophile from this direction is less likely to occur due to the higher activation energy for this process.
c. The "C-H"-function would still be a "C-H"- function because NaBH4 serves as the hydride source. The product would possess an "O-H" or an "O-D" group because CD3OH and D2O were used as proton sources. The product distribution in terms of endo and exo product would not change significantly compared to a.
d. The reaction will be more diastereoselective because a bulkier hydride source (i.e., LiBH (sec.-butyl)3) is used. However, the student would have to use diethyl ether or THF as a solvent in the reaction because the reagent would react very quickly with a protic solvent and is less polar as well. In addition, the reaction would require much more of the reducing reagent because the reagent only bears one hydride.
3. Online quiz (no answer key, make an appointment with the instructor if you have questions)
4. a.
Diethyl ether is a poor choice as solvent because sodium borohydride is ionic and only dissolves in polar solvents (i.e.,
water, methanol, ethanol). Thus, the reaction mixture would be
heterogenous. However, camphor is weakly polar because of the large
hydrocarbon portion of the molecule (log Kow=2.38).
Methanol is used as solvent in Chem 30BL, which is a compromise in terms
of polarity to dissolve both compounds.
b. The initial
dissolution of camphor favors the reduction of the carbonyl group over
the reaction of the sodium borohydride with the the protic solvent,
methanol.
NaBH4 + 4 CH3OH ------> Na[B(OCH3)4] + 4 H2
c. The gentle reflux facilitates the dissolution of the reducing reagent. The reaction also proceeds much faster than at room temperature. However, the diastereoselectivity will be a little lower than at low temperatures. :-(
d. The ice-cold water is added to precipitate the organic compounds (isoborneol, borneol and camphor), while the ionic sodium salts like NaBH4, Na(B(OMe)4, Na(B(OH)4, etc.) remain in solution. In addition, it also helps to hydrolyze the tetraalkyl borate (NaB(OR)4) and to quench the excess of the sodium borohydride. The chilling of the reaction mixture is needed to control the reaction better, which tends to foam very heavily otherwise.
e. The crude is dissolved in diethyl ether before anhydrous magnesium sulfate is added. Since the product has an hydroxyl group, it tends to adsorb on the polar drying agent as well. Thus, it is very important to minimize the amount of drying agent used in this step to reduce loss due this absorption. The dry solution is transparent and some drying agent is free-flowing in the solution upon swirling.
f. The gas chromatograph in Chem 30BL utilizes a capillary column. Thus, the capacity of this column is very low. Only a small amount of a very dilute sample (1-2 mg/mL diethyl ether) should be introduced in order to observe the proper separation. A higher concentration will cause an overlap of the peaks making the quantitation difficult, if not impossible, with simple methods.
g. The specific optical rotation of the sample is [a]= -20.625o (= -0.165/(0.040 g/5.00 mL)). Thus, the sample contains 80.7 % of (-)-isoborneol and 19.3 % of borneol (using the equations given in lecture) and the diastereoselectivity is 61.4 % (=80.7 %-19.3 %).
h. The carbonyl peak at ν=1744 cm-1 show disappear.
Instead, a broad peak for the hydroxyl group (ν=3200-3600 cm-1)
and a strong peak at ν=1069 cm-1 for the C-OH function.
i. In order to get a strong signal, the compound has to have good
contact with the ATR crystal. This is the case for liquid because they
spread out evenly. In cases of solids, the contact is general very poor.
Thus, pressure has to be applied to the solid to improve the contact.