Meeting 2 Problem set - CONDENSED KEY
1. a. Thiamine is used as the catalyst for the benzoin condensation in Chem 30BL. The reaction of the thiamine hydrochloride with sodium hydroxide leads to the formation of the free thiamine. The color of the solution will change from colorless to dark yellow to pale yellow in the end.
Th-H+Cl- + OH- ----> Th + H2O + Cl-
b. The thiamine hydrochloride dissolves well in water but poorly in 95 % ethanol. Thus, the salt is dissolved in water before 95 % ethanol is added to lower the polarity of the solution to allow for the benzaldehyde to dissolve.
c. The reaction mixture is stored in a closed 6-dram vial to prevent the aerial oxidation of benzaldehyde to benzoic acid, which would be the dominant reaction upon exposure to air.
d. In order to determine the yield, the following procedure is useful.
- List all compounds in the reaction mixture: 95 % ethanol, water, thiamine hydrochloride, sodium hydroxide, benzaldehyde
Identify the function of the individual compounds i.e., solvent (water, 95 % ethanol), catalyst (thiamine hydrochloride, sodium hydroxide=both precursors) and reactant (benzaldehyde).
- Outline chemical equation
2 mol benzaldehyde (A) -----> 1 mol benzoin (B)
- Determine the moles of limiting reagent
Since benzaldehyde is the only reagent here, it is the limiting reagent by definition.
nA = (2.80 mL * 1.044 g/mL) /106.12 g/mol = 27.5 mmol
nB(expected) = 27.5 mmol/2 =13.8 mmol (because of molar ratio in the chemical equation)
- Determine the yield for product
nB= 2.30 g / 212.24 g/mol =10.8 mmol
yield =10.8 mmol / 13.8 mmol * 100 % = 78.7 %
Note that catalysts are usually used in ~1-10 mole % compared to the limiting reagent!
2. Video/Online Quiz (no key posted!)
If you have any questions, please see the instructor after 1 pm on Friday of the week.
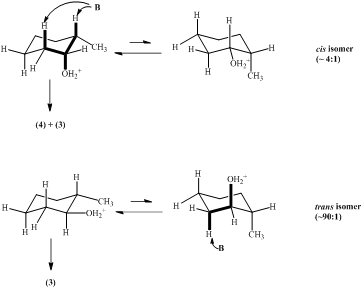
3. a. The protonation of the hydroxyl function leads to the formation of water as leaving group. In the case of the cis conformer, the intermediate reacts out of the preferential conformer. In the case of the trans conformer, the elimination takes place out of the less favorable conformer. Thus, the cis alcohol is much more reactive than the trans alcohol.

b. Amberlyst A15 is a polymer-based catalyst. It can be regarded as polymeric form of p-toluenesulfonic acid. The advantage of this catalyst is that it can be recycled, which is usually not possible for concentrated phosphoric acid or other mineral acids. Since it is a solid acid, it is much safer to handle than the other acids. In addition, concentrated sulfuric acid leads to a lot of decomposition (as indicated by the black color of the reaction mixture).
c. The reaction underlies an equilibrium, which displays a low equilibrium constant (estimated: K<10 at 100 oC). In order to obtain a decent yield, the student distills the product (methylcyclohexenes) and the byproduct (water) out of the reaction mixture, which causes the system to produce more of these products taking advantage of the Le Châtelier principle.
d. The initial reflux is needed to produce the methylcyclohexenes and water, which are later removed from the mixture by distillation. If the distillation was started immediately or too early, most of the 2-methylcyclohexanol would be distilled before it could react. :-(
e. Rule 9 states
The vapor pressure of solids or liquids will increase significantly when these compounds are heated. Thus, a pressure will build up in a closed container without a pressure release. Recall, that one mole of liquid water occupies a volume of V=18 mL while 1 mol of steam occupies a volume of V=30,600 mL (100 oC), about 1700 times more. Note that even a gas will expand upon heating.
f. The concentration of the solution for GC analysis should be 1-2 mg/ml in diethyl ether to prevent an overload of the column and the mass spectrum detector.
g. The GC/MS printout will provide information about the composition (gas chromatogram) and the identity (mass spectrum) of the compounds in the mixture.
4. Data analysis
4.1. The cations are formed by a series of hydride and alkyl shifts
that lead to the ring expansion or contraction.
4.2. The more substituted alkenes are more stable than the less substituted ones. The heats of formation of the cyclohexenes are more negative than the cyclopentenes, which is a result of the lower ring strain in the cyclohexenes. The tertiary cations are more stable than the secondary cations and the primary cation. The six-membered ring cations are more stable than the five-membered ring cations, which display some ring strain.
4.3. The most stable cations are most likely the intermediate for the formation of the alkene. 1-Methylcyclohexene, the major product, can be formed from the initial secondary cation and from the tertiary cation, which is the most likely intermediate. 3-Methylcyclohexene, one of the minor products, can be formed from the initial cation and from a rearranged, secondary cation. Methylenecyclohexane, another minor product, can only be formed from the tertiary cation. The five-membered ring based cations display a higher energy and therefore contribute less to the formation of products.
4.4. The calculated data is adequate in terms of magnitude for the neutral compounds considering the low level model (AM1). The absolute energies for the intermediates are a too high and therefore do not allow for exact prediction of the product ratio. However, it can predict the trends correctly, i.e., major product.