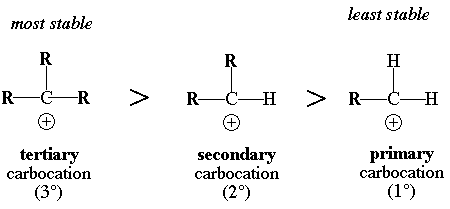
The more R groups (alkyl groups) attached to the carbocation, the more stable it is.
Thus,

The reason why tertiary carbocations are more stable than primary carbocations is that R groups inductively donate electron density to the electron deficient carbon of the carbocation. The more R groups the more electron density that is donated to help stabilize the electron deficient carbon.