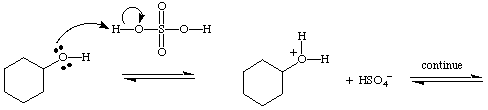
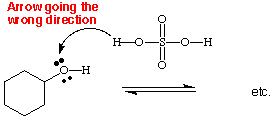
A mechanism shows the movement (or flow) of electrons by using arrows. For example, in the first step of the mechanism for the dehydration of cyclohexanol the arrow is going from the lone pair of electrons on the oxygen to the H+ ion. Make sure the arrow is going in the correct direction as shown in example #1. Incorrectly drawn is example #2 .
example #1.
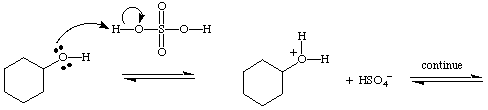
example #2. (INCORRECT)
II. Acid Catalyzed Dehydrations
Sulfuric acid (H2SO4) and phosphoric (H3PO4) acid are the most commonly used acid catalyst in dehydration of alcohols. The dehydration ultimately results in the Elimination of water (a H2O unit). In general, the elimination pathway is either unimolecular (E1) or bimolecular (E2) in nature. Primary alcohols favor the E2 pathway. Secondary and tertiary alcohols favor the E1 pathway.
III. E1 Pathway
The E1 pathway results in loss of a water unit and formation of an intermediate carbocation. The steps of a E1 pathway are summarized below:
1. protonation of OH group
2. Loss off protonated OH group (i.e., H2O) and formation of a carbocation.
3. Rearrangement of the carbocarbocation may occur.
4. Elimination of the adjacent proton leaving behind its sigma electrons. Formation of the alkene results.