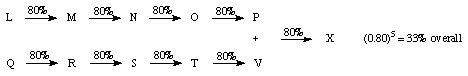Synthesis of Tetraphenylnaphthalene
(Meeting 9, July 24/25, 2002)
THERE WILL BE AN IN-LAB QUIZ!!!
I. Multi-step synthesis
A. Background - Synthesis of biologically active compounds (i.e., pharmaceutical and agricultural industries) usually requires many steps. It is not uncommon for a reaction sequence to require more than 20-30 steps for the target compound. Multi-step syntheses require considerable research and development, which can easily climb into the millions of dollars. Following is are the basic steps required to bring a financially prospective compound to fruition:
- Target (is it interesting?)
- Test its biological activity (animal studies)
- Test it on human
- FDA approval
- Optimize synthesis for mass production
- Marketing
Bottom line is the compound will not be produced unless it is financially rewarding.
B. Shotgun Approach
1. Synthesis
a. Robotic synthesis (extractions)
b. Combinatorial Chemistry - technique used in the mass production of closely related compounds. These related compounds are known as a "library".
2. Screening of chemical library - once these groups of related compounds (i.e., a library) are amassed then systematic testing takes place in order to determine efficacy (effectiveness).
a. Virtual Screening (computational)
b. Genomic Screening
Ultra high throughput genomic screening
1980's 30 /week
1999 350,000/week
2000 600,000/week
2003 10,000,000/week
microscale is critical to keep cost down. If it cost 1¢ per screen then 10,000,000 screenings cost $$$!
3. From Clinical Studies to marketing.
1 out every 10,000 make it to clinical studies
1 out of 300 put into clinical studies are successful.
These targets must be produced from commercially available compounds (e.g., Aldrich, Merck, etc.). Choosing the most efficient and cost effective route is necessary for a company's financial survival. There are two main strategies used in a multi-step synthesis:
C. Strategy (approach)
1. Linear
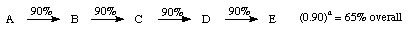
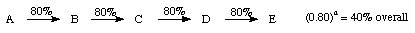
Some published syntheses are 40 steps or more! (0.80)40 = 0.013 % ouch!
(Thus one starts with a kilogram of material and ends up with a milligram of material -- if your skill and luck does well that year)
Problems: (1) low overall yield and (2) time consuming both of which cost $$$
One way to reduce these problems is by using a convergent strategy
2. Convergent - rather than building the target molecule one piece at a time, two fairly equal sized fragments are built concurrently and then in the last step "tied" together.
Linear Route:
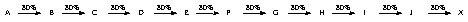
overall yield of 11 % (baring any disasters!)
Convergent Route:
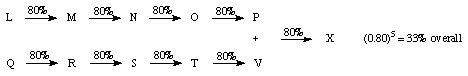
Another advantage is, that if an intermediate is used up then the time (and resources) required remaking this intermediate is considerably less when compared to the linear route.
In our lab, this step will complete a four-step synthesis starting from Benzaldehyde to tetraphenylnaphthalene.
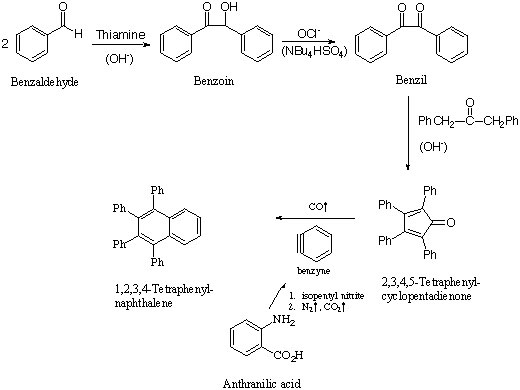
Synthesis of 1,2,3,4-tetraphenylnaphthalene:
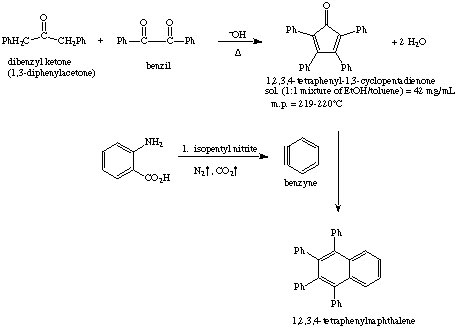
Generation of Benzyne:
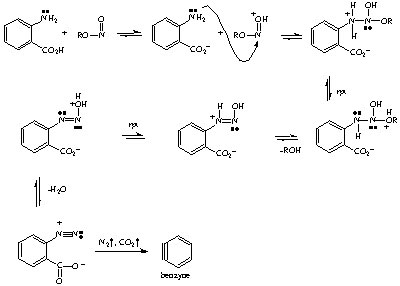
Final step:
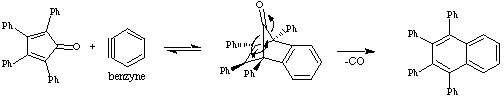
Procedure for Diels-Alder Reaction -Benzyne Reaction
Place 0.20 g of tetraphenylcyclopentadienone, 0.09 g of anthranilic acid and 2.5 mL of 1,2-dimethoxyethane in a 10-mL round bottom flask and attach a air condenser. In a hood, transfer 0.15 mL of isopentyl nitrite (isoamyl nitrite, mw = 117.2, d = 0.875 g/mL) and 1 mL of 1,2-dimethoxyethane to a 5-mL conical vial. Cap the vial and replace the lid on the reagent bottle as soon as possible.
Attention: Isopentyl nitrite is a powerful heart stimulant. Avoid breathing. Dispensing of this compound should take place in the hood. Isopentyl nitrite must be refrigerated when not in use. Restopper bottle after usage in order to minimize contact with air (isopentyl nitrite is both air and light sensitive).
Bring the mixture to a gentle boil and then add the isopentyl nitrite solution through the top of the condenser over a 60 second period. Use a few drops of 1,2-dimethoxyethane to rinse the vial and add this solution to the reaction mixture. Continue to boil this mixture until a color change is observed (goes from purple to yellow orange. If a color change is not observed within 10 minutes, add a drop or two of pure isopentyl nitrite). After the color change, cool the reaction mixture to room temperature and transfer to a beaker containing 10 mL of water and 4 mL of methanol while stirring vigorously. Collect the solid by filtration (Note: Additional product may precipitate out in the filter flask.) Collect this material and add it to the other solid. Weigh the crude product.
Recrystallize the product from isopropyl alcohol in a 50 mL Erlenmeyer flask. Initially, add 5-10 mL of isopropyl alcohol and then bring to a boil. Slowly add hot isopropyl alcohol until the crude product has completely dissolved (or nothing appears to dissolve anymore). The crude tetraphenyl-naphthalene is very slow to dissolve- so be patient and stir vigorously. Once the crude product dissolves, allow cooling to room temperature (~5 minute) then cool in an ice-bath. After several minutes of cooling, scratch side with a glass rod to induce crystal growth (or use a seed crystal). The product slowly crystallizes over a 30 minute period. Collect the product on a Hirsch funnel and wash the crystals with a small amount of ice-cold isopropyl alcohol.
Determine the melting point of the dry product. Pure 1,2,3,4-tetraphenylnaphthalene exhibits a double melting point (196-199 °C and 203-205 °C). If your melting point is 196-199 °C then remove capillary and allow product to solidify. Redetermine the melting point. Obtain an IR (KBr) und UV spectrum of the final product.
Obtain the UV-Vis Spectrum of the final product in acetonitrile (conc~1*10-4 mol/L).
Combine the postlabs of meeting 8 and meeting 9 into one report. In the postlab compare the UV spectrum and the IR spectrum of tetraphenylnaphthalene and tetraphenylcyclopentadienone.