Review the following topics: recrystallization theory, vacuum filtration, GC chromatography
DO NOT DISCARD ANYTHING INTO THE WASTE CONTAINER BEFORE YOU ISOLATED YOUR FINAL PRODUCT.
2. Experiment
Due to the moisture sensitivity of the Grignard reagent it is very important that you dry all your glassware very thoroughly (this means no visible water droplets!). All bottles should be closed if not in use to keep the moisture out of the solvents and reagents.
The setup should look like this:
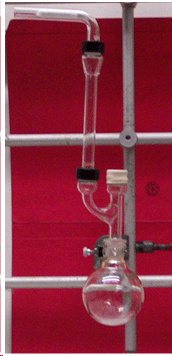
The round-bottomed flask (or three-necked flask) has to be clamped at the neck and not at the bulb part!
The rubber septum has to be folded over in order to provide a good seal. Just placing them loosely on the top will not do the same job.
The drying agent used in the lab is CaCl2 in its granular form. Powders are not useful here since they are clogging the drying tube up entirely after a proper packing. The drying agent has to be sandwiched between two cotton plugs. Make sure to push the outer cotton plug in as far as possible so that any air going into the setup has to pass the drying agent.
About 12 mmol of Grignard reagent are needed for the reaction. The entire setup should be assembled before the TA dispenses the reagent with the syringe. The lab support will provide a 1.4 M solution of MeMgBr in toluene/THF this quarter.
During the work-up, the 10% sulfuric acid solution has to be added slowly especially in the beginning. Why?
After the organic and the aqueous layer are separated the first time (be careful here!), the aqueous layer has to be extracted with diethyl ether again. Why?
The combined organic layers are extracted with sodium bicarbonate. Why?
The drying step shoudl employ the minimum quantity of anhydrous MgSO4. Why?
In most cases, two recrystallizations will be required in order to get a relatively pure compound. In rare cases, one recrystallization will be enough to remove the unreacted benzoin. Do not to place the solution in an ice bath. What are you trying to remove at this point? How do you know if you are done purifying the sample?
Submit two GC samples (~3 mg/mL) for evaluation. (The samples should not contain any visible amounts of water since this will ruin the column and the separation will be rather poor as well.) One from the crude product (after the workup before starting the recrystallization) and a second one once you isolated your final product in order to evaluate its purity. The bar code number (1-999) has to be recorded. Sign in your name and the vial numbers in the list that the TA provides. The spectra can be picked up from the instructors office usually in the afternoon of the following day.
A little of the crude product should be left behind for TLC (meeting 9).
The product should not be disposed of after the first meeting since it will be used it again during the next meeting to obtain a TLC spectrum.
3. Things to think about
a. Which compounds can be found in the crude product?
b. Why are 1,2-dibromides not good starting materials for Grignard reactions?
c. Why is it important not to use too much drying agent in this experiment?
d. How many signals do you expect to see for the product in the 1H and 13C-NMR spectrum?
e. Why is the description of the Grignard reagent as "RMgX" not entirely correct?
f. Why are ethers most commonly used for Grignard reactions?
g. What are alkyl iodides very good substrates for the formation of Grignard reagents?