updated last
Answer Key Extra Credit Project (DHPM)
1. Many dihydropyrimidinones (DHPM) have pharmacological and therapeutic properties. They display many interesting biological effects such as antiproliferative, antiviral (Nitractin), anti-tumour, anti-inflammatory, antibacterial, anti-histaminic, antifungal, antitubercular, anticancer (i.e., (S)-Monastrol) and anti-analgesic activities.
2. The reaction does not use a solvent during the reaction and only very little during the purification. Some sunlight is used as heat source because the grinding in the mortar proved to be insufficient for the reaction to occur at a reasonable rate (as described in the literature). The solvent-free condition makes this reaction an environmentally benign procedure because it reduces problems that are associated with the use of large quantities of organic solvents such as safety, waste, cost, handling and pollution. In this reaction, only water and ethanol are used.
3. The p-toluenesulfonic acid (p-CH3C6H4SO3H)
is used as catalyst in the reaction. Initially,
urea and benzaldehyde undergo an
acid-catalyzed formation of a N-acyliminium
ion. The cation reacts with a CH-acidic compound (via its enol form) to
yield the ureide, which undergoes an acid-catalyzed cyclization and
dehydration to form a racemic mixture of 4-phenyl–substitited
3,4-dihydropyrimidin-2-one.
4. The mixture has to be mixed thoroughly before it is stored in the sunlight to overcome the barriers associated with solvent-free reactions. Only part of the urea and the catalyst dissolve in the liquids. The vial has to be closed well to prevent the oxidation of benzaldehyde.
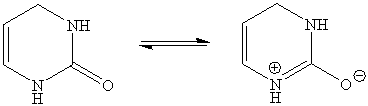
5. The planarity of the central six-membered ring is due to the resonance of the alkene function and the amide function (only the center ring is shown below).

6. A solvent mixture of 95 % ethanol and acetone (1:3) is used as solvent for the recrystallization. The compound dissolves well in most polar solvents like methanol, ethanol or DMSO but less in medium (i.e., acetone) and low polarity solvents (i.e., CHCl3).
7. The 1H-NMR spectrum of the compound displays nine signals: two signals for the amide protons (7.8 and 9.2 ppm, 2 "s", 2 H), three signals for the benzene ring (~7.2-7.5 ppm, d/t/t, 5 H), one signal for the benzylic hydrogen (~5.2 ppm, s, 1 H), one signal for the methyl group on the alkene function (2.3 ppm, s, 3 H) and two signals for the ethyl function (1.1 ppm, t, 3 H; 3.9 ppm, q, 2 H).