updated last
Answer Key Extra Credit Project (DHPM)
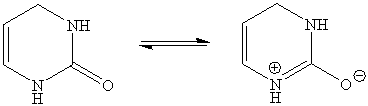
1. The planarity of the central six-membered ring is due to the resonance of the alkene function and the amide function (only the center ring is shown below). Most of the atoms in the ring display formally a sp2-hybridization.

2. The amide functions, which are very polar, form very strong hydrogen bonds with the carbonyl oxygen atoms of the neighboring molecule that leads to a double-strand of molecules (four hydrogen bonds per molecule, see X-ray structure in the reader). These strong intermolecular forces lead to a high melting point.
3. The p-toluenesulfonic acid (p-CH3C6H4SO3H,
TSA)
is used as catalyst in the reaction. Initially,
urea and 4-nitrobenzaldehyde undergo an
acid-catalyzed formation of a N-acyliminium
ion. The cation reacts with a CH-acidic compound (via its enol form) to
yield the ureide, which undergoes an acid-catalyzed cyclization and
dehydration to form a racemic mixture of 4-(4-nitrophenyl)–substitited
3,4-dihydropyrimidin-2-one.
4. The
mixture has to be mixed thoroughly before it is stored in the sunlight
to overcome the barriers associated with solvent-free reactions. Only
part of the urea, the 4-nitrobenzaldehyde and the catalyst dissolve in
the liquids (ethyl acetoacetate). The vial has
to be closed well to prevent the oxidation of the benzaldehyde.
5. A 6-dram vial is used for the reaction. The regular black cap is
replaced by a compression cap and a flat Teflon septum that is pushed
inwards a little (as shown on the right side below).

6. A mixture of acetone and ethyl acetate is used for recrystallization. The crude is first dissolved in acetone. Ethyl acetate is added to lower the polarity of the solvent causing the precipitation of the product.
7. The 1H-NMR spectrum of the compound displays eight signals: two amide signals (7.89 ppm (s, 1H), 9.35 ppm (s, 1H)), two signals in the aromatic range (7.48 ppm (d, 2H), 8.17 ppm(d, 2H) and four signals in the aliphatic range (5.25 ppm (s, 1H), 3.95 ppm (q, 2H), 2.25 ppm (s, 3 H) and 1.05 ppm (t, 3H)).