updated last
Answer Key Extra Credit Project (DHPM)
1. The reaction does not use a solvent during the reaction and only very little during the purification. Some sunlight is used as heat source because the grinding in the mortar proved to be insufficient for the reaction to occur at a reasonable rate (as described in the literature). The solvent-free condition makes this reaction an environmentally benign procedure because it reduces problems that are associated with the use of large quantities of organic solvents such as safety, waste, cost, handling and pollution. In this reaction, only water and ethanol are used.
2. The p-toluenesulfonic acid (p-CH3C6H4SO3H)
is used as catalyst in the reaction. Initially,
urea and 4-chlorobenzaldehyde undergo an
acid-catalyzed formation of a N-acyliminium
ion. The cation reacts with a CH-acidic compound (via its enol form) to
yield the ureide, which undergoes an acid-catalyzed cyclization and
dehydration to form a racemic mixture of 4-(4-chlorophenyl)–substitited
3,4-dihydropyrimidin-2-one.
3. The
mixture has to be mixed thoroughly before it is stored in the sunlight
to overcome the barriers associated with solvent-free reactions. Only
part of the urea and the catalyst dissolve in the liquids. The vial has
to be closed well to prevent the oxidation of the benzaldehyde.
4. A mixture of 95 % ethanol and acetone (1:3) is used for the
recrystallization. The DHPM dissolves well in polar solvents like
alcohols, but less in medium and low polarity solvents like acetone and
chloroform. The impurities (i.e., benzaldehydes) are less polar than the
product and dissolve well in these solvents.
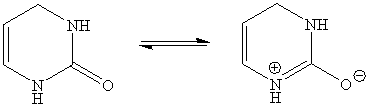
5. The planarity of the central six-membered ring is due to the resonance of the alkene function and the amide function (only the center ring is shown below).

7. The 13C-NMR spectrum of the compound displays twelve signals: two carbonyl carbons (150-170 ppm), six aromatic/alkene carbons (100-150 ppm), four aliphatic carbons (10-80 ppm).