last updated
1. Experimental
1.1. Molybdenum pentachloride (prepared in a group of 2-3 students)
This part of the experiment will be usually carried out by the two (or three) students. The reaction has to be upscaled a little bit (~0.5 g of Mo-powder for each student) in order to obtain enough of the product for everybody in the group.
The equipment that you will be using here is pretty expensive (quartz tube and quartz boat). Don't break it!
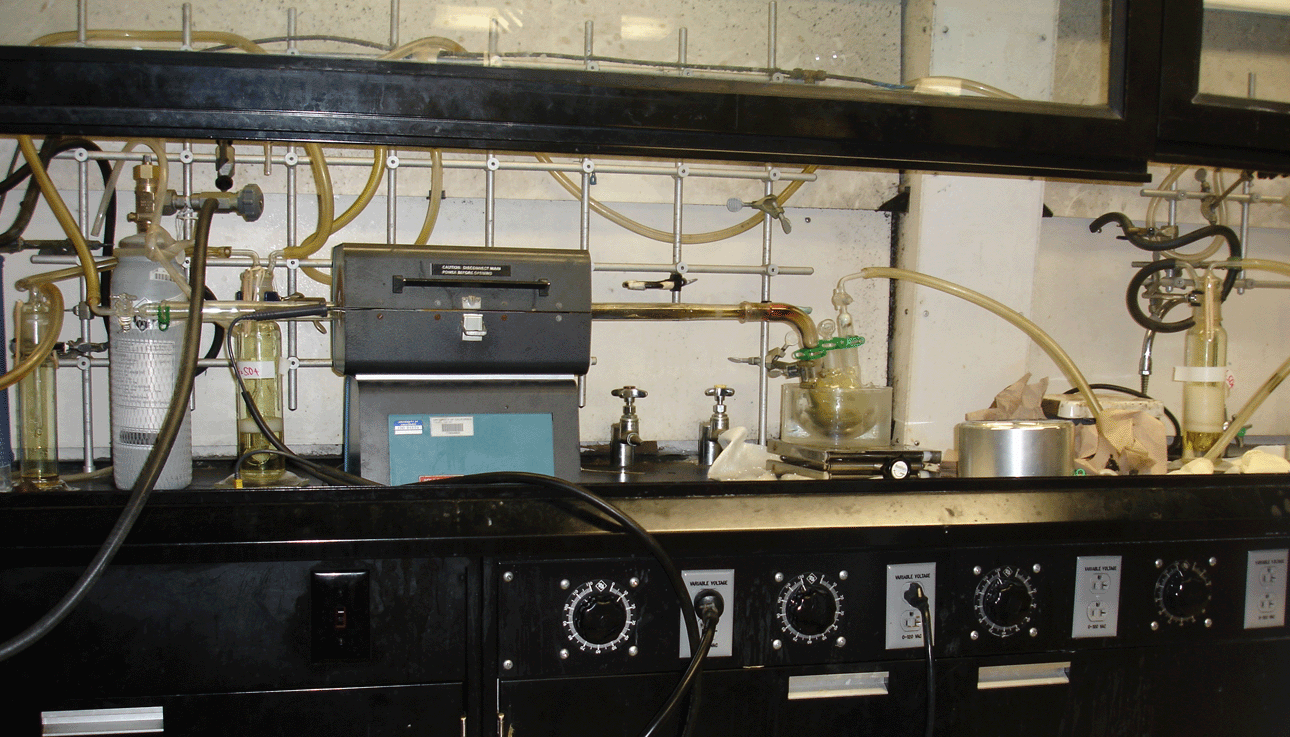
The setup (shown below, many thanks to Sandy Nguyen, a former student and later TA in the class, for the picture) for the chlorination reaction takes up a lot of time. Therefore, it would be a good idea to do this during the lab section prior to the day the chlorination is scheduled for the group. It is advisable to flame-dry the setup as well already and then seal it off. The bubbler at the end of the setup has to contain concentrated sulfuric acid and not mineral oil, because this tends to polymerize relatively quickly.

The initial step is a reduction of the the Mo-powder with hydrogen gas at ~1000 oC. It is important that the furnace is not heated up too fast. The heating has to be carried out under hydrogen and not under nitrogen (Why?).
After 30-60 minutes, the setup is cooled to about 200-300 oC. The system is purged with carbon dioxide (Why?) before chlorine gas is introduced to the Mo-metal. Usually a dark red vapor forms immediately if the metal is sufficiently active. If this does not happen, the Mo-metal has to be heated until the reaction initiates. During the reaction, the product is driven into the Schlenk flask using a heat gun.
Warning: The stopcock plug at the end of the setup will clog up easily if the flow rate of the chlorine gas is too high. One way to avoid this is to place a cotton ball loosely in the gas adapter. The overpressure will generate leaks (usually at the connection of the quartz tube to the bent adapter) which will introduce air to the system. The color will change to dark green. Another possibility is to use a relatively big three-necked flask, which allows the product to settle down better.
After all the Mo-powder reacted and all the product is sublimed into the Schlenk flask (or three-necked flask), the setup is allowed to cool down. The chlorine gas is replaced by nitrogen gas to purge the setup. The Schlenk flask is disconnected and then evacuated. The evacuated flask is transferred into the glove box. The crude is weight and then ~3-4 g are transferred to a Pyrex tube. This tube is evacuated and then placed in a furnace (T~250 oC) to separate the oxychlorides from the MoCl5. The dark blue compound obtained in this step is separated and used for the solid state reaction.
After the reaction is completed, the setup has to be disassembled and cleaned immediately because it is usually very difficult to do so after a couple of days. The grease hardens quickly from the exposure of the chlorine gas and the MoCl5.
Note: A two-stage regulator has to be used for the hydrogen tank. In case of ammonia, chlorine and carbon dioxide, a needle valves is usually sufficient. Make sure to read appendix F (Handlling of gas cylinders) before starting the project. The needle vales have to be thoroughly cleaned afterwards. The chlorine and ammonia tank have to be properly closed and securely stored.
1.2. Sodium sulfide (prepared individually)
Please be aware that a liquified gas is used here as a solvent. Together with the fact that sodium metal is used in the reaction as well there is a high potential for accidents if the reaction is not properly carried out. The cooling bath should be below -50 oC at all times (except when the ammonia is removed at the end of the reaction!). The reaction vessel has to be connected to a bubbler at all times to prevent a pressure buildup.
Sodium metal is stored under mineral oil. This can be removed by dipping a piece in hexane or petroleum ether. The metal is very soft and can easily be cut with a knife or razor blade. The outer oxide layer should be removed. If a little more or less than 0.92 g of sodium metal is used, the quantity of sulfur has to be adjusted accordingly.
Generally, about 30 mL of liquid ammonia are sufficient to run the reaction. The color is initially dark blue (sodium metal dissolved in ammonia) and will change to orange and the white (or very pale yellow). If the color remain orange, a little more Na-metal should be added.
After the color changed, the cooling bath is removed and the ammonia allowed to evaporate. The remaining fine powder has to be transferred a the Pyrex tube (in glove box). The Pyrex tube is evacuted and the compound then annealed at temperatures below 250 oC (not higher!!!) over night .
1.3. Molybdenum sulfide (performed individually)
The reaction vessel is a stainless steel container with a lid and a filament (Ni-Chrome) wire on the inside. One should make sure that all pieces required in the reaction are transferred into the glove box together with a Variac (as power supply). It might also be advisable to have a spare filament as well. The filament has to go all the way down to the bottom of the vessel.
Approximately 0.5 g of MoCl5 and 0.36 g of Na2S are mixed well in a small container i.e. vial or small beaker. The obtained mixture is transferred into the steel vessel, which is then and then closed tightly. The filament has to touch the mixture. The contacts on the top are connected to the Variac. The power is slowly ramped to setting '10' on the Variac. This is usually enough to initiate the reaction. After a minute, the bomb is carefully opened to check if a reaction actually took place. If not, if the ignition sequence is repeated with a slightly higher setting after checking if the filament is still intact..
Once the reaction took place, the mixture is allowed to cool and transferred into a beaker. The workup is done in the hood (outside the glove box). The mixture is washed with methanol (carefully!), water, methanol and carbon disulfide (highly flammable! It has to be collected in a separate waste bottle!) and then dried in vacuo.
2. Characterization
X-ray powder diffraction (ask your TA for help with that)
3. Hints to the questions
ad 1: What is the student trying to remove here?
ad 4: How is chlorine gas produced in industry?
ad 6: Which compounds can form if the reaction employs more than 0.5 mole equivalents of sulfur?
ad 8: What exactly happens when you place the tube in a furnace
that is heated to 250
oC?