
last updated
Porous materials like zeolites have been used for a long time in
industrial application as catalysts or support of catalysts in the
petroleum industry, in water purification, in gas separation, or as a
drying agent (molecular sieves). They display porous structures that are
based on a Si/Al-oxide
structures that contain additional cations i.e., Na+, K+,
Mg2+, Ca2+, etc. that modify their properties like
their Lewis acidity or the size of their pores/channels significantly.
Due to the large channels in the structures, they usually display very
low densities compared to other minerals
(SiO2: 2.65 g/cm3, Al2O3:
4.00 g/cm3, CaO: 3.34 g/cm3). Some zeolites like
clinoptilolite
((Na,K,Ca)2-3Al3(Al,Si)2Si13O36·12
H2O,
r=2.15
g/cm3),
stilbite (NaCa4(Si27Al9)O72·28
H2O,
r=2.15
g/cm3) and natrolite (Na2Al2Si3O10·2
H2O,
r=2.25
g/cm3) occur in nature. ZSM-5 (named after Zeolite
Sconoy Mobile, NanAlnSi96–nO192·16
H2O (0<n<27),
r=0.72
g/cm3,) is an artificial zeolite that is used for the
isomerization of meta-xylene or ortho-xylene to para-xylene and as
support for the copper-based oxidation of ethanol too

Recently, metal-organic frameworks (MOF) have garnered a lot of
attention because of their unique properties. They consist of a metal
ion and an organic ligand that links the metal ions together into larger
arrays. Many dicarboxylic acids (i.e., oxalic acid, malonic acid,
succinic acid, glutaric acid, terephthalic acid), tricarboxylic acid
(i.e., citric acid, trimesic acid) or azoles (i.e., 1,2,3-triazole,
pyrrodiazole) are used as linker.
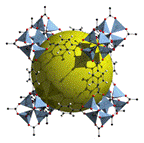
MOF-5 (Zn4O(1,4-benzenedicarboxylate)3,
r=0.13-0.20
g/cm3) consists of tetrahedral [Zn4O]6+
units that are linked
together with 1,4-benzene-dicarboxylate units. The opening in the
structure is 9.3-13.8 Å depending on the orientation of the ring. MOF-5
can store a significant amount of hydrogen at low temperature (77 K: 7.1
wt % (40 bar), 10 wt % (100 bar)). While the hydrogen storage capacity
in decent at 77 K (66 g/L), its ability to store hydrogen at room
temperature is significantly lower (9.1 g/L), which limits its use as
hydrogen storage medium.
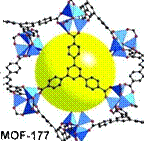
MOF-177 (Zn4O(1,3,5-benzenetribenzoate)2) also
consists of tetrahedral [Zn4O]6+ units are linked
by large, triangular tricarboxylate ligands. Its hydrogen storage
capacity is similar to the one of MOF-5 (77 K: 7.1 wt % (40 bar), 11.4
wt % (78 bar)). MOF-200 and MOF-210 display a little bit higher uptake
of hydrogen at low temperature and are able to deliver 3 % at 100 bar at
room temperature.
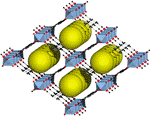
MIL-53 ([Al(OH)] (1,4-benzenedicarboxylate) (1,4-benzenedicarboxylic
acid)0.7,
r=0.4
g/cm3) consists linear chains of [AlO4(OH)2]
octahedra that are linked together with 1,4-benzene-dicarboxylate units.
Like many other MOFs, it is obtained by the reaction of the metal
nitrate with the dicarboxylic acid under hydrothermal conditions.
Recently, the iron, chromium and scandium analog of MIL-53 have been
prepared as well.
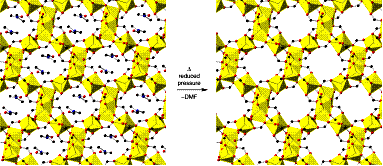
In this experiment, the students will synthesize
a-magnesium
formate, which also displays
MOF–type properties as well, in solvothermal fashion. Since the
synthesis of
a-Mg3(O2CH)6
is carried out in N,N-dimethylformamide,
the solvent will be included into the porous structure
(see picture on the left). Upon heating under reduced pressure,
the guest can be removed without disrupting the framework as the crystal
structure of the two compounds demonstrates.
Other molecules (i.e., THF, Et2O, MeOH, EtOH, C6H6,
C7H8, C6H12, etc.) may or
may not be used to fill the voids of the guest-free structure depending
on their size.
Experiment:
1.
The drum vial is closed with a flat septum (Teflon side down, pushed
inwards) and a compression cap.
2. The four compounds obbtained in this project will be characterized by infrared and 1H-NMR and 13C-NMR spectrum spectroscopy (in D2O)
3. The SPARTAN PART aims to determine the size of the molecules to be included.
Hints for the questions:
ad 1: What exactly happens during the heating part?
ad 4: Do you expect the structure to change after the inclusion of a solvent molecule?
ad 7: What determines the temperature needed to remove the solvent molecules?