Introduction
Nitric oxide (NO) is a colorless gas that is thermodynamically unstable. Due its odd number of electrons, the compound is paramagnetic. The unpaired electron is located in a π*-orbital. Due to the formal triple bond, the molecule possesses a small dipole moment (μ=0.158 D) in which the nitrogen atom possesses a partial negative charge (60 % spin density on nitrogen based on EPR). The N-O bond length (115 pm) corresponds with a bond order of about 2.5 (double bond: 118 pm, triple bond: 106 pm).
Nitric oxide is a by-product of combustion of substances in the air (i.e., in automobile engines, fossil fuel power plants). In both cases, it is quickly oxidized to form nitrogen dioxide, which is removed using a catalytic converter. Nitric oxide is also produced naturally during the electrical discharges of lightning in thunderstorms. However, the direct reaction of nitrogen with oxygen requires temperatures in excess of 2000 oC, which is not practical for a large-scale synthesis. Industrially, the oxidation of ammonia at 850 oC with platinum as catalyst leads to the formation of nitric oxide.
4 NH3 + 5 O2 ------> 4 NO + 6 H2O
Nitrosyl chloride is formed in a mixture of hydrochloric acid and nitric acid also known as aqua regia.
HNO3 + 3 HCl ------> ClNO + Cl2 + 2 H2O
In metal nitrosyl complexes, nitric oxide (NO) is bonded to a metal. The nitrosyl ligand can act as a one-electron or a three-electron donor. The nitrosyl cation (:N≡O:+) can use its lone pair on the nitrogen atom to form a bond to the metal atom like its isoelectronic counterparts the carbonyl (:C≡O:) and the cyanide (:C≡N:-). As carbonyl ligands, various degrees of π-back bonding are observed (NO+ is a stronger π-acceptor than CO):
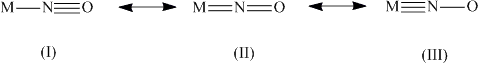
Increasing strength of the π-back bond strengthens the M-N bond and weakens the N-O bond. In extreme cases, both bonds can be characterized as double bonds (II). In cases in which the M-N-O angle is close to 180o, the M-N bond is usually relatively short. If the backbonding effect is weak, the angle decreases significantly ( (M-N-O)= ~120o) and the ligand can be described as “NO-“ as it is observed in the cobalt complexes below. The Enemark-Feltham notation is used to describe the number of d-type electrons present in a given metal nitrosyl complex. Complexes with "d+π"-in excess of six often tend to have bent NO ligands
Experiment
In the lab, an iron nitrosyl complex will be synthesized by the reaction of ferrous sulfate with nitrous acid in the presence of a dithiocarbamate ligand. The reaction of the nitrite with sulfuric acid generates the nitrous acid (HNO2), which is reduced by Fe2+ to yield nitric oxide, which reacts with the iron aquo complex to form [Fe(H2O)5(NO)]2+. The addition of the dithiocarbamate ion leads to the formation of the dark-green Fe(NO)dtc2.
2 NaNO2 + 2 FeSO4 + 3 H2SO4 -------> Fe2(SO4)3 + 2 NaHSO4 + 2 H2O + 2 NO
In the second part, the product is reacted with iodine in absolute ethanol to yield the brown cis-FeI(NO)dtc2. The structures of both compounds are investigated using infrared spectroscopy, EPR and NMR spectroscopy.
Hints for the pre-lab questions:
ad 2: See above
ad 3: What determines the solubility of a compound in a solvent?
ad 4: What is the "magic number" for transition metal complexes?
ad 7: Which method can be used to separate gases?