
The π orbital of ethylene has two orbital lobes (one shown in the red and the other in blue), and one orbital node (the plane which contains the atoms).
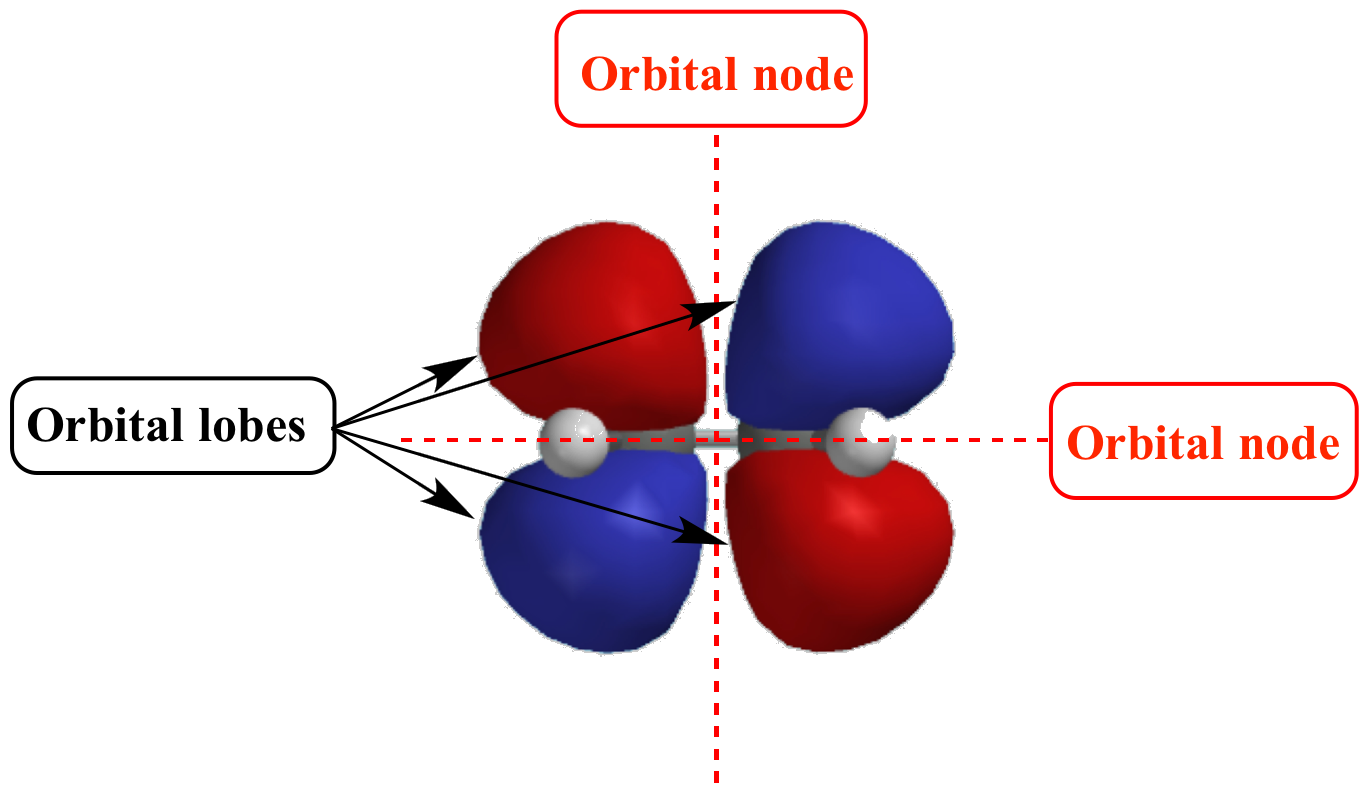
The π* orbital of ethylene has four orbital lobes (one above and below the molecular plane on each carbon), and two orbital nodes (the plane which contains the atoms, and another plane, perpendicular to the first, and bisecting the molecule).