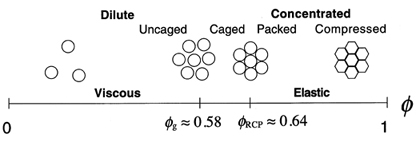
| We
study extremely fine metastable emulsions known as nanoemulsions because the droplets are typically less than 100 nm. Nanoemulsions are not
equilibrium mesophases (i.e. lyotropic liquid crystalline phases known as
"microemulsions") that can exhibit swollen spherical micellar phases over a
relatively narrow range of droplet volume fractions. Instead, once formed by extreme emulsification, metastable nanoemulsions have droplet volume fractions that can be controlled over an extremely wide range through osmotic concentration and dilution. |
A highly concentrated
nanoemulsion is a strongly
elastic biliquid foam.
Due to the extremely small
size of the droplets, concentrated
nanoemulsions
are nearly transparent.
|